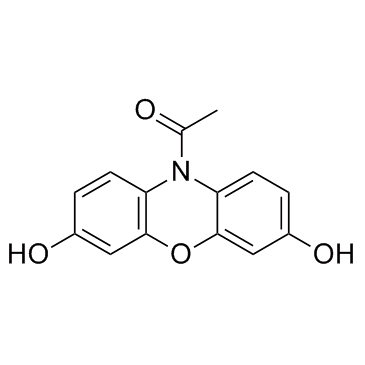
119171-73-2
| Name | ADHP [10-Acetyl-3,7-dihydroxyphenoxazine] |
|---|---|
| Synonyms |
10-Acetyl-10H-phenoxazine-3,7-diol
1-(3,7-Dihydroxy-10H-phenoxazin-10-yl)ethanone 1-(3,7-dihydroxyphenoxazin-10-yl)ethanone MFCD00467861 N-acetyl-3,7-dihydroxyphenoxazine 10H-Phenoxazine-3,7-diol, 10-acetyl- Ampliflu™ Red Amplex red Ethanone, 1-(3,7-dihydroxy-10H-phenoxazin-10-yl)- 10-Acetyl-3,7-dihydroxyphenoxazine ADHP |
| Description | ADHP is a fluorogenic peroxidase substrate (λex=530 nm, λem=590 nm). |
|---|---|
| Related Catalog | |
| In Vitro | To obtain the parameters Km and kcat for Compound I, two independent methods are used. Initially, the oxidation of ADHP using the injector functionality built-in to the fluorescence plate reader is studied. The auto-injector dispenses the H2O2 to initiate the reaction, as a means of generating a set of progress curves. Analysis for MPO-mediated oxidation of ADHP gives a Km of 31±4 μM and the kcat of 186± 6 s−1.The kobs also increases over the experimental range of ADHP concentrations from 1 to 80 μM and for the converse experiment holding substrate constant over 3 to 45 nM MPO. The apparent second order rate constant obtain from the slope of kobs against ADHP concentration Kappon is 2.1±0.2 mM/s[1]. |
| Kinase Assay | ADHP, 4-ABAH, 2-ABAH, 4-BAH, 4-FBAH, 4-NBAH, 4-TFMBAH, 3-DMABAH, NaN3 and isoniazid are dissolved in DMSO and subsequently diluted into assay buffer. The final concentration of DMSO in the reaction is less than 0.5 % (v/v), which does not affect fluorescence of the oxidized ADHP product 7-hydroxyl-3H-phenoxazin-3-one (resorufin). Reactions of ADHP (20 μM) are incubated with MPO (2.8 nm) in assay buffer and initiated by the addition of 1/10th volume H2O2 from a serial dilution basin. To determine the effect that the simplest benzoic acid hydrazide inhibitor or its analog 4-TFMBAH has on the heme catalytic ability of MPO, MPO (1.2 μM) is incubated for 10 min with different concentrations of BAH inhibitor (0, 0.025, 0.25, 2.5, 12.5 and 25 mM) with ADHP (40 μM) and timing of the reaction is measured following addition of H2O2 (20 μM) ADHP. All reactions are measured in assay buffer at room temperature. Samples of 20 μL are added to non-reducing sample loading buffers, and then loaded without prior heating and resolved by 4-15% gradient SDS-polyacrylamide gel electrophoresis[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 618.6±55.0 °C at 760 mmHg |
| Molecular Formula | C14H11NO4 |
| Molecular Weight | 257.241 |
| Flash Point | 327.9±31.5 °C |
| Exact Mass | 257.068817 |
| PSA | 70.00000 |
| LogP | 0.89 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.689 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |