7729-23-9
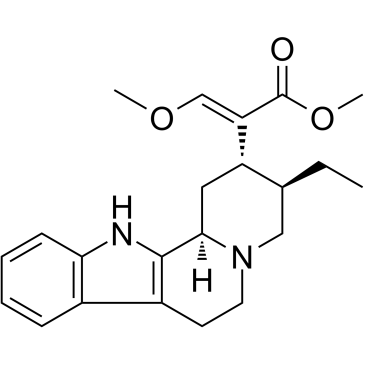
| Name | methyl (E)-2-[(2S,3R,12bR)-3-ethyl-1,2,3,4,6,7,12,12b-octahydroindolo[2,3-a]quinolizin-2-yl]-3-methoxyprop-2-enoate |
|---|---|
| Synonyms |
Indolo[2,3-a]quinolizine-2-acetic acid, 3-ethyl-1,2,3,4,6,7,12,12b-octahydro-α-(methoxymethylene)-, methyl ester, (αE,2S,3R,12bR)-
Methyl (3β,16E)-16-(methoxymethylene)corynan-17-oate Hirsutine Methyl (3β,16E)-17-methoxycoryn-16-en-16-carboxylate methyl (3β,16E)-16-(methoxymethylidene)corynan-17-oate |
| Description | Hirsutine, an indole alkaloid of Uncaria rhynchophylla, exhibits anti-cancer activity. Hirsutine induces apoptosis and is a potent Dengue virus inhibitor exhibiting low cytotoxicity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Hirsutine remarkably reduces the viability of MCF-7 and MDA-MB-231 cells in a time- and dose-dependent manner with IC50 values of 447.79 and 179.06 μM, respectively. In the MDA-MB-231 cells, Hirsutine induces apoptosis and depolarization of MMP, releases Cyt C from mitochondria, and activates caspase 9 and caspase 3[2]. |
| In Vivo | Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in human lung cancer cells[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 531.7±50.0 °C at 760 mmHg |
| Melting Point | 101℃ |
| Molecular Formula | C22H28N2O3 |
| Molecular Weight | 368.469 |
| Flash Point | 275.4±30.1 °C |
| Exact Mass | 368.209991 |
| PSA | 54.56000 |
| LogP | 3.96 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.613 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|