64228-79-1
| Name | atracurium |
|---|---|
| Synonyms |
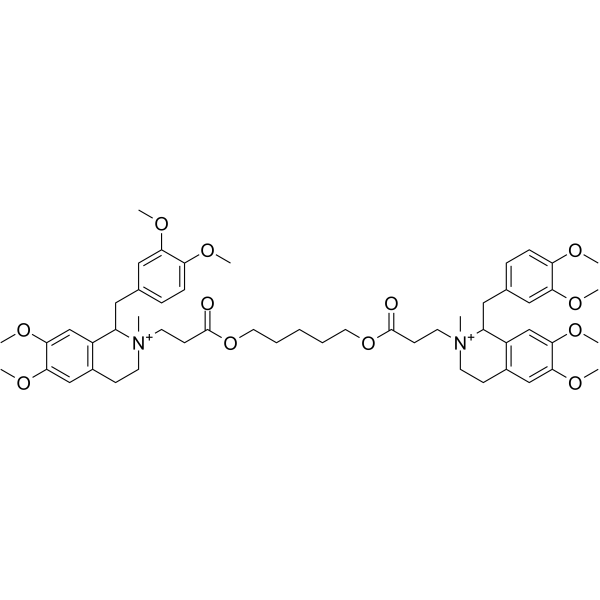
5-[3-[1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-2-ium-2-yl]propanoyloxy]pentyl 3-[1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-2-ium-2-yl]propanoate
2,2'-{1,5-Pentanediylbis[oxy(3-oxo-3,1-propanediyl)]}bis[1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolinium] Isoquinolinium, 2,2'-[1,5-pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl- Atracurium 2,2'-{Pentane-1,5-diylbis[oxy(3-oxopropane-3,1-diyl)]}bis[1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolinium] UNII-2GQ1IRY63P Atracurium Dibesylate |
| Description | tracurium (BW-33A free acid) is a potent, competitive and non-depolarizing neuromuscular blocking agent.Atracurium also is an AChR receptor antagonist. Atracurium induces bronchoconstriction and neuromuscular blockade. Atracurium promotes astroglial differentiation[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| In Vitro | Atracurium (10 µM; 72 h) promotes astroglial but not neuronal differentiation in HSR040622 and HSR040821 cells[4]. Atracurium (10 µM; 48 h) reduces tumor engraftment and increases survival of mice xenotransplanted with ex-vivo treated GSCs[4]. Atracurium (2.4 µM; 120 min) induces a complete fade of the tetanic contraction while only slightly affected the twitch in rat extensor digitorum longus muscle cells[5]. Cell Proliferation Assay[4] Cell Line: glioblastoma stem (GSC) cells Concentration: 3, 10, 20 µM Incubation Time: 72 h Result: Increased the percentage of GFP-positive cells in a dose-dependent manner from 5.3% in DMSO to 15.4%, 81.1%, and 86.8% in 3 μM, 10 μM, and 20 μM, respectively. |
| In Vivo | Atracurium (1, 5, 10, 20, 50 mg/kg; i.v.) induces bronchoconstriction in DBA/2 and SJL mice[2]. Atracurium (4.8 mg/kg; i.v.) induces neuromuscular blockade in rats[3]. Animal Model: 5-12 weeks, 15-20 g male mice[2] Dosage: 1, 5, 10, 20, 50 mg/kg Administration: I.v. Result: Induced bronchoconstriction and Atracurium-induced airway hyperresponsiveness in DBA/2 mice was eliminated in a dose-dependent manner by pretreatment with atropine or pancuronium. Animal Model: 290 ± 30 g Male Sprague±Dawley rats (60 mg/kg heat-killed Corynebacteriumparvum for i.v.)[3] Dosage: 4.8 mg/kg Administration: I.v. Result: Induced neuromuscular blockade in Corynebacteriumparvum-injected rats. |
| References |
| Melting Point | 185-194ºC |
|---|---|
| Molecular Formula | C53H72N2O12++ |
| Molecular Weight | 929.144 |
| Exact Mass | 928.507446 |
| PSA | 126.44000 |
| LogP | 1.04 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|