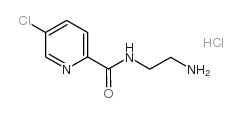
103878-83-7
| Name | N-(2-aminoethyl)-5-chloropyridine-2-carboxamide,hydrochloride |
|---|---|
| Synonyms |
Lazabemide hydrochloride
Ro 19-6327/001 Huwentoxin IV Tempium Lazabemide monohydrochloride Lazabemide HCl Ro 19-6327 |
| Description | Lazabemide hydrochloride (Ro 19-6327 hydrochloride) is a selective, reversible inhibitor of monoamine oxidase B (MAO-B) (IC50=0.03 μM) but less active for MAO-A (IC50>100 μM). Lazabemide inhibits monoamine uptake at high concentrations, the IC50 values are 86 μM, 123 μM and >500 μM for noradrenalin, serotonin and dopamine uptake, respectively. Lazabemide can be used for the research of parkinson and alzheimer′s disease[1]. |
|---|---|
| Related Catalog | |
| Target |
MAO-B:0.4 nM (IC50) |
| In Vitro | The in vitro binding characteristics of both radiolabeled inhibitors revealed them to be selective, high-affinity ligands for the respective enzymes. KD and Bmax values for 3H-Ro 19-6327 in rat cerebral cortex are 18.4 nM and 3.45 pmol/mg protein, respectively[1]. The IC50 values for lazabemide are: 86 μM for NA uptake; 123 μM for 5HT uptake; > 500 μM for DA uptake, respectively[1]. . Lazabemide (5 μM) inhibits human MAO-B and MAO-A with IC50 of 6.9 nM and >10 nM, respectively. And it inhibits rat MAO-B and MAO-A with IC50of 37 nM and >10 μM, respectively ina enzymatic assay[2]. Lazabemide differs from L-deprenyl in their ability to induce release of endogenous monoamines from synaptosomes. Thus, Lazabemide (500 μM) induces a greater 5 HT release than does L-deprenyl, but is less effective than L-deprenyl in releasing DA. On the contrary, lazabemide was almost completely inactive on either 5 HT and DA release[2]. Lazabemide (250 nM) results in a clear inhibition of DOPAC formation, while does not increase the accumulation of newly-formed DA in those tubular epithelial cells loaded with 50 microM L-DOPA[3]. |
| In Vivo | Lazabemide (3 mg/kg) attenuates ichemia reperfusion-induced hydroxyl radical generation and pretreatment with Lazabemide showed decreased DOPAC levels in comparison with those of their respective vehicle-treated control groups[4]. |
| References |
| Boiling Point | 397.4ºC at 760mmHg |
|---|---|
| Molecular Formula | C8H11Cl2N3O |
| Molecular Weight | 236.09800 |
| Flash Point | 194.2ºC |
| Exact Mass | 235.02800 |
| PSA | 68.01000 |
| LogP | 2.31670 |
| Vapour Pressure | 1.59E-06mmHg at 25°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|