396129-53-6
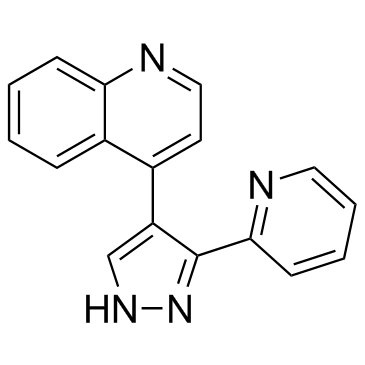
| Name | 4-(3-Pyridin-2-yl-1H-pyrazol-4-yl)quinoline |
|---|---|
| Synonyms |
4-[3-(2-Pyridinyl)-1H-pyrazol-4-yl]quinoline
Quinoline, 4-[3-(2-pyridinyl)-1H-pyrazol-4-yl]- 4-(5-pyridin-2-yl-1H-pyrazol-4-yl)quinoline LY 364947 4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]quinoline LY364947 LY-364947 |
| Description | LY-364947 is a potent ATP-competitive inhibitor of TGFβR-I with IC50 of 59 nM, and exhibits 7-fold selectivity over TGFβR-II. |
|---|---|
| Related Catalog | |
| Target |
IC50: 59 nM (TGFβR-I) |
| In Vitro | LY-364947 is an ATP competitive and tight-binding inhibitor, inhibiting phosphorylation of P-Smad3 by TGFβR-I Kinase with Ki of 28 nM. LY-364947 inhibits in vivo Smad2 phosphorylation within the NMuMg cells with IC50 of 135 nM. LY-364947 reverses TGF-β-mediated growth inhibition in NMuMg cells with IC50 of 0.218 μM. LY-364947 potentiates the xVent2-lux BMP4 response in NMuMg cells by 30% at concentrations as low as 0.25 μM. LY-364947 (2 μM) prevents TGF-β-induced epithelial−mesenchymal transition in NMuMg cells[1]. LY-364947 (3 μM) induces expression of Prox1 and LYVE-1 in almost all HDLECs after 24 hours[2]. LY-364947 promotes nuclear export of Foxo3a, with low Smad2/3 and high Akt phosphorylation levels in leukaemia-initiating cells. LY-364947 (< 20 μM) suppresses leukaemia-initiating cells colony-forming ability after co-culture with OP-9 stromal cells[3]. |
| In Vivo | LY-364947 (1 mg/kg, i.p.) accelerates lymphangiogenesis, as evidence by significantly increasing the LYVE-1-positive areas in a mouse model of chronic peritonitis. LY-364947 (1 mg/kg, i.p.) significantly increases the LYVE-1-positive areas in tumor tissues in tumor xenograft models using BxPC3 pancreatic adenocarcinoma cells[2]. LY-364947 (25 mg/kg) increases p-Akt and decreases nuclear Foxo3a in leukaemia-initiating cells in CML-affected mice[3]. |
| Kinase Assay | The IC50 of LY-364947 at different enzyme concentrations are determined by the filter-binding assay. Typically, 40 μL reactions in 50 mM HEPES at pH 7.5, 1 mM NaF, 200 μM pKSmad3(-3), and 50 mM ATP containing a titration of each inhibitor with concentrations of 1600, 800, 400, 200, 100, 50, 25, and 0 nM are incubated at 30°C for 30 min. The IC50 is calculated using a nonlinear regression method with GraphPad Prism software. The binding type is determined by plotting the correlation between enzyme concentrations and IC50 values. |
| Animal Admin | BALB/c nude mice 5 to 6 weeks of age are used in the assay. Parental, or VEGF-C- or TGF-β1-expressing tumor cells (5×106) in 100 μL PBS are implanted subcutaneously into male nude mice and allowed to grow for 2 to 3 weeks to reach proliferative phase, before initiation of TβR-I inhibitor administration. TβR-I inhibitor LY-364947, dissolved in 5 mg/mL in DMSO and diluted with 100 μL PBS, or the vehicle control, is injected intraperitoneally at 1 mg/kg, 3 times a week for 3 weeks. Excised samples are directly frozen in dry-iced acetone for immunohistochemistry. Frozen samples are further sectioned at 10-μm thickness in a cryostat and subsequently incubated with primary and secondary antibodies. Samples are observed using a confocal microscope. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 490.8±45.0 °C at 760 mmHg |
| Molecular Formula | C17H12N4 |
| Molecular Weight | 272.304 |
| Flash Point | 268.7±15.1 °C |
| Exact Mass | 272.106201 |
| PSA | 54.46000 |
| LogP | 2.35 |
| Appearance | white to beige |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.700 |
| Storage condition | Store at RT |
| Water Solubility | DMSO: soluble2mg/mL, clear |
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335-H400 |
| Precautionary Statements | P261-P273-P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T,N |
| Risk Phrases | 25-36/37/38-50/53 |
| Safety Phrases | 26-45-60-61 |
| RIDADR | UN 2811 6.1/PG 3 |
| HS Code | 2933990090 |
|
~% 
396129-53-6 |
| Literature: Sawyer, J. Scott; Anderson, Bryan D.; Beight, Douglas W.; Campbell, Robert M.; Jones, Michael L.; Herron, David K.; Lampe, John W.; McCowan, Jefferson R.; McMillen, William T.; Mort, Nicholas; Parsons, Stephen; Smith, Edward C. R.; Vieth, Michal; Weir, Leonard C.; Yan, Lei; Zhang, Faming; Yingling, Jonathan M. Journal of Medicinal Chemistry, 2003 , vol. 46, # 19 p. 3953 - 3956 |
|
~% 
396129-53-6 |
| Literature: Sawyer, J. Scott; Anderson, Bryan D.; Beight, Douglas W.; Campbell, Robert M.; Jones, Michael L.; Herron, David K.; Lampe, John W.; McCowan, Jefferson R.; McMillen, William T.; Mort, Nicholas; Parsons, Stephen; Smith, Edward C. R.; Vieth, Michal; Weir, Leonard C.; Yan, Lei; Zhang, Faming; Yingling, Jonathan M. Journal of Medicinal Chemistry, 2003 , vol. 46, # 19 p. 3953 - 3956 |
|
~% 
396129-53-6 |
| Literature: Sawyer, J. Scott; Anderson, Bryan D.; Beight, Douglas W.; Campbell, Robert M.; Jones, Michael L.; Herron, David K.; Lampe, John W.; McCowan, Jefferson R.; McMillen, William T.; Mort, Nicholas; Parsons, Stephen; Smith, Edward C. R.; Vieth, Michal; Weir, Leonard C.; Yan, Lei; Zhang, Faming; Yingling, Jonathan M. Journal of Medicinal Chemistry, 2003 , vol. 46, # 19 p. 3953 - 3956 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |