209481-20-9
| Name | SB 271046 hydrochloride |
|---|---|
| Synonyms |
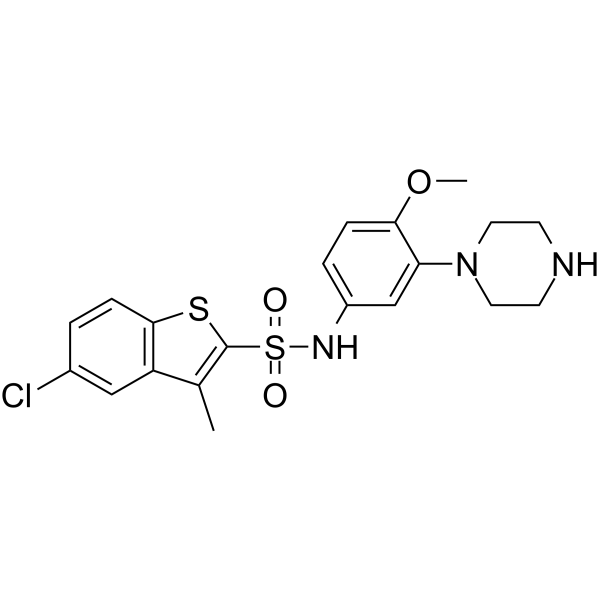
5-Chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-methyl-1-benzothiophene-2-sulfonamide hydrochloride (1:1)
Benzo[b]thiophene-2-sulfonamide, 5-chloro-N-[4-methoxy-3-(1-piperazinyl)phenyl]-3-methyl-, hydrochloride (1:1) 5-Chloro-N-[4-methoxy-3-(1-piperazinyl)phenyl]-3-methyl-1-benzothiophene-2-sulfonamide hydrochloride (1:1) Benzo[b]thiophene-2-sulfonamide (5-chloro-N-[4-methoxy-3-(1-piperazinyl)phenyl]-3-methyl SB271046 |
| Description | SB 271046 is a potent, selective and orally active 5-HT6 receptor antagonist with a pKi of 8.92-9.09. SB 271046 show >200-fold selective for the 5-HT6 receptor over other receptors, binding sites and ion channels. SB 271046 has anticonvulsant activity[1]. |
|---|---|
| Related Catalog | |
| Target |
5-HT6 Receptor:8.92-9.09 (pKi) |
| In Vitro | In functional studies on human 5-HT6 receptors SB 271046 competitively antagonized 5-HT-induced stimulation of adenylyl cyclase activity with a pA2 of 8.71[1]. |
| In Vivo | SB 271046 produces an increase in seizure threshold over a wide-dose range in the rat maximal electroshock seizure threshold (MEST) test, with a minimum effective dose of ⩽0.1 mg/kg p.o. and maximum effect at 4 h post-dose. The level of anticonvulsant activity achieved correlated well with the blood concentrations of SB 271046 (EC50 of 0.16 μM) and brain concentrations of 0.01-0.04 μM at Cmax[1]. |
| References |
| Density | 1.400 |
|---|---|
| Boiling Point | 664.3ºC at 760 mmHg |
| Melting Point | 240-241℃ (DEC.) |
| Molecular Formula | C20H23Cl2N3O3S2 |
| Molecular Weight | 488.45100 |
| Flash Point | 355.5ºC |
| Exact Mass | 487.05600 |
| PSA | 107.29000 |
| LogP | 6.43170 |
| Storage condition | Desiccate at RT |