103300-74-9
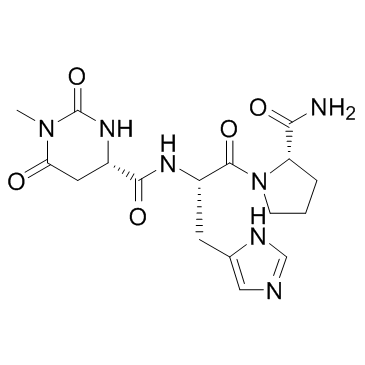
| Name | Taltirelin |
|---|---|
| Synonyms |
Taltirelin
(-)-N-[[(S)-Hexahydro-1-methyl-2,6-dioxo-4-pyrimidinyl]carbonyl]-L-histidyl-L-prolinamide L-Prolinamide, N-[[(4S)-hexahydro-1-methyl-2,6-dioxo-4-pyrimidinyl]carbonyl]-L-histidyl- N-{[(4S)-1-Methyl-2,6-dioxohexahydro-4-pyrimidinyl]carbonyl}-L-histidyl-L-prolinamide (4S)-N-[(2S)-1-[(2S)-2-carbamoylpyrrolidin-1-yl]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]-1-methyl-2,6-dioxo-1,3-diazinane-4-carboxamide N-{[(4S)-1-Methyl-2,6-dioxohexahydropyrimidin-4-yl]carbonyl}-L-histidyl-L-prolinamide TA-0910 Taltirelin Acetate |
| Description | Taltirelin(TA0910), a novel orally active TRH analogue, binds to rat brain TRH receptors in vivo.Target: OthersTaltirelin(TA0910), a novel orally active TRH analogue, binds to rat brain TRH receptors in vivo. Effects of taltirelin hydrate (CAS 103300-74-9, TA-0910), a novel thyrotropin-releasing hormone (TRH) analog, on the cerebral monoamine systems, especially the release and turnover of dopamine (DA) in rat brain were compared with those of TRH by intraperitoneal administration. Taltirelin hydrate (1-10 mg/kg) increased the extracellular levels of DA and its metabolites, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the nucleus accumbens and corpus striatum for 3 h in a microdialysis study. taltirelin hydrate possesses not only an enhancing effect on DA release, but also a stimulating effect on the monoamine system [1]. Taltirelin selectively bound to TRH receptors and increased the spontaneous motor activity by a single administration, suggesting that the motor effect of taltirelin is mediated by TRH receptors [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 72-75° |
| Molecular Formula | C17H23N7O5 |
| Molecular Weight | 405.408 |
| Exact Mass | 405.176056 |
| PSA | 170.59000 |
| LogP | -4.09 |
| Index of Refraction | 1.612 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|