405911-17-3
| Name | GW3965 hydrochloride |
|---|---|
| Synonyms |
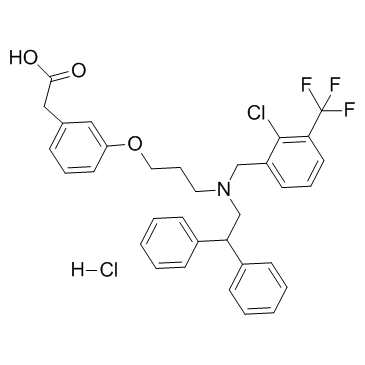
2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methyl-(2,2-diphenylethyl)amino]propoxy]phenyl]acetic acid,hydrochloride
Benzeneacetic acid, 3-[3-[[[2-chloro-3-(trifluoromethyl)phenyl]methyl](2,2-diphenylethyl)amino]propoxy]-, hydrochloride (1:1) [3-(3-{[2-Chloro-3-(trifluoromethyl)benzyl](2,2-diphenylethyl)amino}propoxy)phenyl]acetic acid hydrochloride (1:1) GW-3965 hydrochloride GW3965 HCl GW3965 (hydrochloride) |
| Description | GW3965 hydrochloride is a potent and selective LXR agonist with EC50s of 190 and 30 nM for hLXRα and hLXRβ , respectively. |
|---|---|
| Related Catalog | |
| Target |
EC50: 190 nM (hLXRα), 30 nM (hLXRβ)[4] |
| In Vitro | GW3965 hydrochloride promotes GBM cell death in vitro with enhanced efficacy in EGFRvIII-expressing tumor cells. GW3965 hydrochloride up-regulates expression of the cholesterol transporter gene ABCA1 and the E3 ubiquitin ligase IDOL and reduces LDLR levels[2]. LXR ligands inhibits platelet aggregation and calcium mobilization stimulated by collagen or CRP. GW3965 hydrochloride (1 or 5 μM) displays a minor inhibitory effect on fibrinogen binding and P-selectin exposure, when platelets are stimulated with 1 μg/mL CRP. But using higher concentrations of GW3965 hydrochloride (10 μM) or T0901317 (40 μM), the levels of fibrinogen and P-selectin on the platelet surface are reduced[3]. |
| In Vivo | GW3965 hydrochloride induces an increase of neuroactive steroids in the spinal cord, the cerebellum and the cerebral cortex of STZ-rats, but not in the CNS of non-pathological animals. GW3965 hydrochloride treatment induces an increase of dihydroprogesterone in the spinal cord of diabetic animals in association with an increase of myelin basic protein expression[1]. GW3965 hydrochloride (40 mg/kg, p.o.) strongly induces ABCA1 expression and reduces LDLR expression, and this is accompanied by 59% inhibition of tumor growth, and a 25-fold increase in GBM cell apoptosis in vivo[2]. GW3965 hydrochloride (2 mg/kg, i.v.) increases bleeding time and modulated platelet thrombus formation in vivo[3]. |
| Cell Assay | Cells are seeded in 96 wells and are treated after 24 hours with different drugs indicated in each experiment in medium containing 1% FBS or lipoprotein deficient serum. Relative proliferation is determined using Cell Proliferation Assay Kit. Cells are incubated 1.5 hrs after adding tetrazolium salt WST-1 [2-(4-iodophenyl)-3- (4-nitrophenyl)-5-(2, 4-disulfo-phenyl)-2H-tetrazolium, monosodium salt] at 5% CO2, 37ºC and the absorbance of the treated and untreated cells are measured using a microplate reader at 420 to 480 nm. Cells seeded in 12 well plates are counted using a hemocytometer, and dead cells are assessed using trypan blue exclusion assays. |
| Animal Admin | Diabetes is induced in two-month-old male rats by a single i.p. injection of freshly prepared STZ (65 mg/kg) in 0.09 M citrate buffer, pH 4.8. Control animals are injected with 0.09 mol/L citrate buffer at pH 4.8. Hyperglycemia is confirmed 48 h after streptozotocin injection by measuring tail vein blood glucose levels using a glucometer OneTouch Ultra2. Only animals with mean plasma glucose levels over 300 mg/mL are classified as diabetic. Glycemia is also assessed before treatment with Ro5-4864 or GW3965 hydrochloride and before death. Two months after STZ injection, diabetic animals are treated once a week with Ro5-4864 (3 mg/kg) or GW3965 hydrochloride (50 mg/kg). Thus, they receive four subcutaneous injections in a month. Control diabetic rats receive 200 μL of vehicle (sesame oil). Four-month-old non-diabetic male rats are injected, following the same experimental schedule, with Ro5-4864, GW3965 hydrochloride or vehicle. Rats are killed 24 h after the last treatment. |
| References |
[3]. Spyridon, Michael., et al. LXR as a novel antithrombotic target. Blood (2011), 117(21), 5751-5761. |
| Molecular Formula | C33H32Cl2F3NO3 |
|---|---|
| Molecular Weight | 618.513 |
| Exact Mass | 617.171143 |
| PSA | 49.77000 |
| LogP | 8.89110 |
| Storage condition | -20℃ |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H318-H413 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| RIDADR | NONH for all modes of transport |