29838-67-3
| Name | astilbin |
|---|---|
| Synonyms |
4H-1-Benzopyran-4-one, 3-[(6-deoxy-α-L-mannopyranosyl)oxy]-2-(3,4-dihydroxyphenyl)-2,3-dihydro-5,7-dihydroxy-, (2R,3R)-
Astilbin Dihydroquercetin 3-rhamnoside Taxifolin 3-rhamnoside 4H-1-Benzopyran-4-one, 3-((6-deoxy-α-L-mannopyranosyl)oxy)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-5,7-dihydroxy-, (2R,3R)- Taxifolin 3-rhamnoside Neoisoastilbin Astilbin (2R-trans)-3-((6-Deoxy-α-L-mannopyranosyl)oxy)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-5,7-dihydroxy-4H-1-benzopyran-4-one 4H-1-Benzopyran-4-one, 3-((6-deoxy-α-L-mannopyranosyl)oxy)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-5,7-dihydroxy-, (2R-trans)- Taxifolin 3-O-rhamnoside Astilbin from Engelhardtia roxburghiana (2R,3R)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-2,3-dihydrochromen-4-one (2R,3R)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-3,4-dihydro-2H-chromen-3-yl 6-deoxy-α-L-mannopyranoside |
| Description | Astilbin, a flavonoid compound, is isolated from the rhizome of Smilax glabra. Astilbin enhances NRF2 activation. Astilbin also suppresses TNF-α expression and NF-κB activation. |
|---|---|
| Related Catalog | |
| Target |
NRF2 TNF-α NF-κB |
| In Vitro | Astilbin is a common dietary flavonoid that can be found in various kinds of herbs and foods such as Smilax Glabra, Sarcandra glabra, grape and red wine. Astilbin markedly inhibits cisplatin-induced cell apoptosis and recovers cell growth. Astilbin significantly decreases reactive oxygen species (ROS) accumulation and alleviates ROS-induced activation of p53, MAPKs and AKT signaling cascades, which in turn attenuates cisplatin-induced HEK-293 cell apoptosis. Astilbin effectively enhances NRF2 activation and transcription of its targeting antioxidant genes to reduce ROS accumulation in cisplatin-induced HEK-293 cells. Astilbin obviously suppresses tumor necrosis factor alpha (TNF-α) expression and NF-κB activation, and also inhibits the expression of induced nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). To measure the effects of Astilbin on the growth of CDDP-treated renal cells, HEK-293 cells are treated with CDDP (100 μM) and/or Astilbin (200 μM). Astilbin treatment significantly improvescell growth in CDDP-induced HEK-293 cells[1]. |
| In Vivo | To explore whether Astilbin improves CDDP-induced nephrotoxicity in vivo, an acute cisplatin nephrotoxic mouse model is established. Single injection of CDDP with 8 mg/kg dose results in notable weight loss compared with control group. However, the phenomenon is significantly alleviated by Astilbin at dose of 50 mg/kg. The mice fed Astilbin alone do not show any obvious alteration in body weight. Similarly, serum creatinine (SCr) and blood urea nitrogen (BUN) are higher in CDDP-treated mice than in control group. Treatment with Astilbin also decreases SCr and BUN levels. To examine the protective effect of Astilbin on CDDP-induced renal histopathological damage, the mouse kidney sections are stained with H&E. The mice in control group and Astilbin treated group have normal kidney morphology, while kidneys in CDDP group show severe damage with tubular degeneration, necrosis and cystic dilatation of the tubules with focal hemorrhages. Administration of Astilbin mitigated kidney injury, resulting in lower histopathological score compared to CDDP group. The apoptosis of renal cells is also detected using TUNEL staining to determine whether Astilbin treatment decreased renal cell apoptosis in CDDP-induced acute nephrotoxic mice[1]. |
| Cell Assay | HEK-293 cells are seeded into 96-well plate with a density of 5 × 104 cells/well and subsequently treated with CDDP, Astilbin (0, 10, 30, 50, 100, 200 and 300 μM) or CDDP+Astilbin for 24 h. After treatments, 20 μL of 5 mg/mL MTT is added to each well. The cells are incubated for additional 4 h at 37°C. Then cell supernatant is abandoned and 100 μL of formanzan is added to each well. The plate is shaken at room temperature for 15 min. Spectrophotometric absorbance is measured by Synergy Microplate Reader at 570 nm[1]. |
| Animal Admin | Mice[1] Male C57BL/6 mice (20-24 g, 8 weeks of age) are used. After acclimation for one week, the experimental mice are randomly divided into 4 groups with 10 animals per group: control group, CDDP group, CDDP+Astilbin group and Astilbin group. The control group and CDDP group are orally administered saline for 10 days; the CDDP+Astilbin group and Astilbin group are orally administered 50 mg/kg Astilbin for 10 days. The CDDP group and CDDP+Astilbin group receive a single intraperitoneal injection of CDDP on the 7th day of the experiment, while control group and Astilbin group receive saline injection on the same day[1]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 801.1±65.0 °C at 760 mmHg |
| Molecular Formula | C21H22O11 |
| Molecular Weight | 450.393 |
| Flash Point | 282.9±27.8 °C |
| Exact Mass | 450.116211 |
| PSA | 186.37000 |
| LogP | 2.97 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.748 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble1mg/mL, clear, colorless |
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H400 |
| Precautionary Statements | P273 |
| Hazard Codes | N |
| Risk Phrases | 50 |
| Safety Phrases | 61 |
| RIDADR | UN 3077 9 / PGIII |
|
~91% 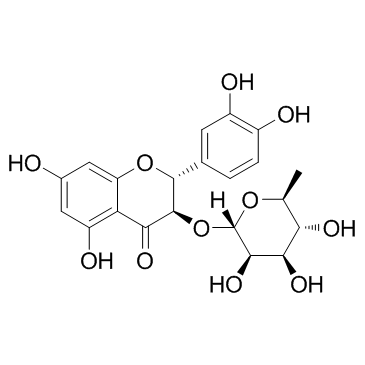
29838-67-3 |
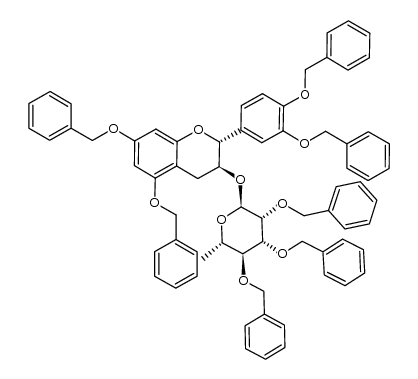
| Literature: SUNTORY LIMITED Patent: EP1260517 A1, 2002 ; Location in patent: Example 6 ; |
|
~% 
29838-67-3 |
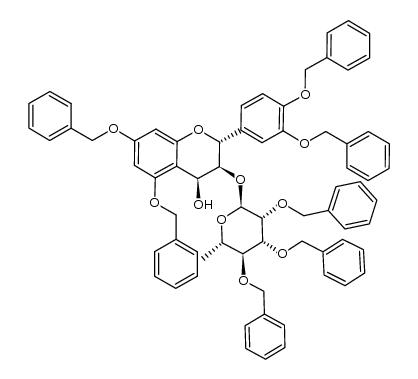
| Literature: Tetrahedron, , vol. 60, # 6 p. 1365 - 1373 |
|
~% 
29838-67-3 |
| Literature: Tetrahedron, , vol. 60, # 6 p. 1365 - 1373 |
|
~% 
29838-67-3 |
| Literature: Tetrahedron, , vol. 60, # 6 p. 1365 - 1373 |
|
~% 
29838-67-3 |
| Literature: Tetrahedron, , vol. 60, # 6 p. 1365 - 1373 |
|
~% 
29838-67-3 |
| Literature: Tetrahedron, , vol. 60, # 6 p. 1365 - 1373 |
| Precursor 6 | |
|---|---|
| DownStream 1 | |