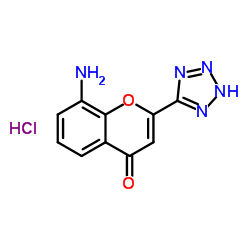
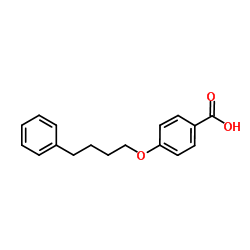
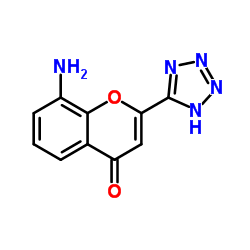
103177-37-3
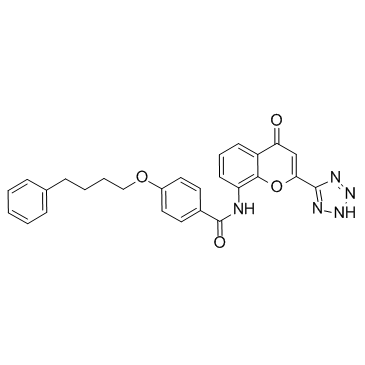
| Name | Pranlukast |
|---|---|
| Synonyms |
ONO RS-411
EINECS 201-616-4 N-[4-Oxo-2-(1H-tetrazol-5-yl)-4H-1-benzopyran-8-yl]-4-(4-phenylbutoxy)benzamide 8-[4-(4-Phenylbutoxy)benzamido]-2-(tetrazol-5-yl)-4H-1-benzopyran-4-one Benzamide, N-(4-oxo-2-(1H-tetrazol-5-yl)-4H-1-benzopyran-8-yl)-4-(4-phenylbutoxy)- N-(4-Oxo-2-(1H-tetrazol-5-yl)-4H-1-benzopyran-8-yl)-p-(4-phenylbutoxy)benzamide N-(4-Oxo-2-(1H-tetrazol-5-yl)-4H-1-benzopyran-8-yl)-4-(4-phenylbutoxy)benzamide N-[4-Oxo-2-(1H-tetrazol-5-yl)-4H-chromen-8-yl]-4-(4-phenylbutoxy)benzamide Pranlukast Benzamide, N-[4-oxo-2-(1H-tetrazol-5-yl)-4H-1-benzopyran-8-yl]-4-(4-phenylbutoxy)- MFCD00864631 4-Oxo-8-(4-(4-phenylbutoxy)benzoylamino)-2-(tetrazol-5-yl)-4H-1-benzopyran 8-[p-(4-Phenylbutoxy)benzoyl]amino-2-(5-tetrazolyl)-4-oxo-4H-1-benzopyran |
| Description | Pranlukast is a highly potent, selective and competitive antagonist of peptide leukotrienes. Pranlukast inhibits [3H]LTE4, [3H]LTD4, and [3H]LTC4 bindings to lung membranes with Kis of 0.63±0.11, 0.99±0.19, and 5640±680 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
LTE4:0.63 nM (Ki) LTD4:0.99 nM (Ki) LTC4:5640 nM (Ki) |
| In Vitro | In the radioligand binding assay, Pranlukast (ONO-1078) inhibits [3H]LTE4, [3H]LTD4, and [3H]LTC4 bindings to lung membranes with Kis of 0.63±0.11, 0.99±0.19, and 5640±680 nM, respectively. The antagonism of Pranlukast against [3H]LTD4 binding is competitive. In functional experiments, Pranlukast shows competitive antagonism against the LTC4- and LTD4-induced contractions of guinea pig trachea and lung parenchymal strips with a pA2 range of 7.70 to 10.71. In the presence of an inhibitor of the bioconversion of LTC4 to LTD4, Pranlukast also antagonizes the LTC4-induced contraction of guinea pig trachea (pA2=7.78). Pranlukast significantly reverses the LTD4-induced prolonged contraction without effect on the KCl- and BaCl2-induced contractions of guinea pig trachea[1]. Oxygen-glucose deprivation (OGD)-induced nuclear translocation of CysLT1 receptors is inhibited by pretreatment with the CysLT1 receptor antagonist Pranlukast (10 μM). Pranlukast protects endothelial cells against ischemia-like injury. The effects of the CysLT1 receptor antagonist Pranlukast and the 5-lipoxygenase inhibitor Zileuton on translocation are also assessed. The results show that Pranlukast, but not Zileuton, inhibits the translocation of the CysLT1 receptor 6 h after OGD[2]. |
| In Vivo | Carrageenan (CAR, 5 mg per mouse) is injected i.p. 24 h before LPS (50 p,g per mouse) is injected i.v. Various doses of Pranlukast (ONO-1078; 40, 20, and 10 mmol/kg), AA-861 (20, 10, and 5 mmol/kg), Indomethacin (40 mmollkg), and the controls are injected s.c. into mice 30 min before they are challenged with 50 p,g of LPS. The maximum soluble doses are 0.6 mmol/mL in 10% DMSO for AA-861 and 1.2 mmol/mL in 10% ethanol for Pranlukast. These solutions are used as the maximum doses for the treatments. The mortality of mice is significantly decreased in AA-861- Pranlukast-treated mice relative to that in the control mice. Pretreatment with CAR (5 mg i.p.) renders the mice more sensitive to the effect of LPS. Although the survival rate of mice treated with each solvent is 20% at 72 h after LPS (50 p,g per mouse) administration, s.c. treatment with AA-861 (20 mmol/kg) or Pranlukast (40 mmol/kg) significantly increases the survival rate after the LPS administration (AA-861, P<0.001; Pranlukast, P<0.01)[3]. |
| Cell Assay | EA.hy926 cells are cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% heat-inactivated fetal calf serum, Penicillin (100 U/mL) and Streptomycin (100 mg/mL). Experiments are conducted 24 h after cells are seeded. OGD is performed. Briefly, the original medium is removed; the cells are washed twice with glucose-free Earle's balanced salt solution (EBSS) and placed in fresh glucose-free EBSS. Cultures are then placed in an incubator containing 5% CO2 and 95% N2 at 37°C for 2 to 8 h. Control cultures are maintained in glucose-containing EBSS under normal conditions. 10 μM Pranlukast, 10 μM Zileuton, a 5-LOX inhibitor or 10 μM Pyrrolidine dithiocarbamate (PDTC), is added to the culture 30 min before OGD exposure and maintained during OGD[2]. |
| Animal Admin | Mice[3] Male ddY mice are used. All mice used are 7 to 8 weeks of age. Endotoxin shock is induced in mice. In brief, CAR (5 mg in 0.5 mL of physiological saline) is injected intraperitoneally (i.p.) as a priming agent 24 h before LPS challenge. LPS (50 p,g in 0.5 mL of physiological saline) is injected intravenously into the tail vein as an inducing agent. The indicated doses of AA-861, Pranlukast (40, 20, and 10 mmol/kg), saline, DMSO, or ethanol are administrated subcutaneously (s.c.) in a volume of 1 mL into the backs of mice 30 min before the LPS provocation. Both drugs are injected s.c., because CAR i.p. pretreatment caused peritonitis. To examine the role of endogenous TNF in CAR pretreated mice, 2×105 U of rabbit anti-TNF-a antibody or normal serum of rabbit in 0.2 mL is injected intravenously (i.v.) before the LPS challenge[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Melting Point | 236-238ºC |
| Molecular Formula | C27H23N5O4 |
| Molecular Weight | 481.503 |
| Exact Mass | 481.175018 |
| PSA | 123.00000 |
| LogP | 3.88 |
| Index of Refraction | 1.681 |
| Storage condition | Store at +4°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | F,C |
|---|---|
| Risk Phrases | R11 |
| Safety Phrases | S16-S26-S36/37/39-S45 |
|
~% 
103177-37-3 |
| Literature: WO2010/2075 A1, ; Page/Page column 17-18 ; |
|
~% 
103177-37-3 |
| Literature: US5874593 A1, ; |
|
~93% 
103177-37-3 |
| Literature: PHARMACOSTECH CO., LTD.; KIM, Jae Won; CHA, Young Gwan; RYU, Hyung Chul; KIM, Sun Joo Patent: WO2010/67913 A1, 2010 ; Location in patent: Page/Page column 11 ; |
|
~% 
103177-37-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 84 - 91 |
|
~% 
103177-37-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 84 - 91 |
|
~% 
103177-37-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 84 - 91 |
|
~% 
103177-37-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 31, # 1 p. 84 - 91 |
|
~% 
103177-37-3 |
| Literature: Synthetic Communications, , vol. 27, # 6 p. 1065 - 1073 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |