3796-63-2
| Name | Teprenone |
|---|---|
| Synonyms |
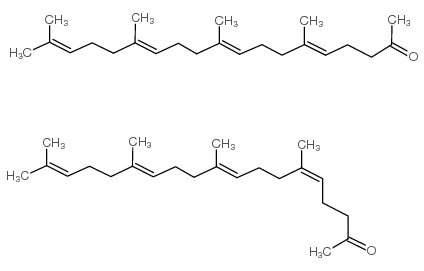
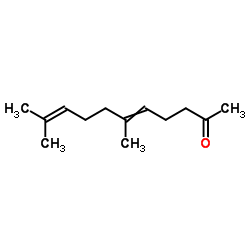
6,10,14,18-tetramethyl-nonadeca-5t,9t,13t,17-tetraen-2-one
E-0671 6,10,14,18-Tetramethyl-nonadeca-5t,9t,13t,17-tetraen-2-on (5E,9E,13E)-6,10,14,18-Tetramethyl-5,9,13,17-nonadecatetren-2-one (5E,9E,13E)-6,10,14,18-tetramethyl-nonadeca-5,9,13,17-tetraen-2-one 5,9,13,17-Nonadecatetraen-2-one,6,10,14,18-tetramethyl-,(E,E,E) 2-Oxo-6,10,14,18-tetramethyl-nonadecatetraen-(5.9.13.17) (E,E,E)-geranylgeranylacetone E,E,E-geranylgeranylacetyl |
| Description | (5E,9E,13E)-Teprenone ((5E,9E,13E)-Geranylgeranylacetone) is an isomer of Teprenone with antiulcer activity. (5E,9E,13E)-Teprenone induces transcriptional activation of HSP genes that may increase gastric mucosal defense at conditions of stress[1]. |
|---|---|
| Related Catalog | |
| In Vitro | (5E,9E,13E)-Teprenone might transiently activate the transcription of HSP70 gene[1]. The heat shock element–binding activity reflects the activation of heat shock factor 1 in response to (5E,9E,13E)-Teprenone[1]. |
| In Vivo | (5E,9E,13E)-Teprenone induces hat sock proteins accumulations in rat gastric mucosa[1]. Animal Model: Male Wister strain rats (≈250 g)[1] Dosage: 200 mg/kg Administration: Oral administration Result: Caused accumulations of all of HSP90, HSP70, HSC70 and HSP60 within 60 minutes. |
| References |
| Density | 0.871g/cm3 |
|---|---|
| Boiling Point | 438.2ºC at 760 mmHg |
| Molecular Formula | C23H38O |
| Molecular Weight | 661.09400 |
| Flash Point | 166.5ºC |
| Exact Mass | 660.58500 |
| PSA | 34.14000 |
| LogP | 15.00260 |
| Index of Refraction | 1.482 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |