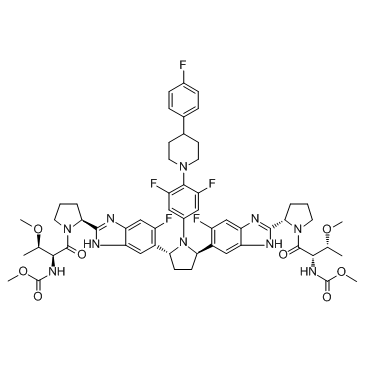
1353900-92-1
| Name | pibrentasvir |
|---|---|
| Synonyms |
Carbamic acid, N,N'-[[(2R,5R)-1-[3,5-difluoro-4-[4-(4-fluorophenyl)-1-piperidinyl]phenyl]-2,5-pyrrolidinediyl]bis[(6-fluoro-1H-benzimidazole-5,2-diyl)(2S)-2,1-pyrrolidinediyl[(1S)-1-[(1R)-1-methoxyethyl]-2-oxo-2,1-ethanediyl]]]bis-, dimethyl ester
methyl {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl}-5-{6-fluoro-2-[(2S)-1-[N-(methoxycarbonyl)-O-methyl-L-threonyl]pyrrolidin-2-yl]-1H-benzodiazol-5-yl}pyrrolidin-2-yl]-6-fluoro-1H-benzimidazol-2-yl}pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl}carbamate pibrentasvir dimethyl ((2S,2'S,3R,3'R)-((2S,2'S)-2,2'-(5,5'-((2R,5R)-1-(3,5-difluoro-4-(4-(4-fluorophenyl)piperidin-1-yl)phenyl)pyrrolidine-2,5-diyl)bis(6-fluoro-1H-benzo[d]imidazole-5,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methoxy-1-oxobutane-2,1-diyl))dicarbamate Methyl {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)-1-piperidinyl]phenyl}-5-(6-fluoro-2-{(2S)-1-[N-(methoxycarbonyl)-O-methyl-L-threonyl]-2-pyrrolidinyl}-1H-benzimidazol-5-yl)-2-pyrrolidinyl]-6-fluoro-1H-benzimidazol-2-yl}-1-pyrrolidinyl]-3-methoxy-1-oxo-2-butanyl}carbamate methyl {(2S,3R)-1-[(2S)-2-{5-[(2R,5R)-1-{3,5-difluoro-4-[4-(4-fluorophenyl)piperidin-1-yl]phenyl}-5-(6-fluoro-2-{(2S)-1-[N-(methoxycarbonyl)-O-methyl-L-threonyl]pyrrolidin-2-yl}-1H-benzimidazol-5-yl)pyrrolidin-2-yl]-6-fluoro-1H-benzimidazol-2-yl}pyrrolidin-1-yl]-3-methoxy-1-oxobutan-2-yl}carbamate |
| Description | Pibrentasvir is a novel and pan-genotypic hepatitis C virus (HCV) NS5A inhibitor with EC50s ranging from 1.4 to 5.0 pM against HCV replicons containing NS5A from genotypes 1 to 6. |
|---|---|
| Related Catalog | |
| Target |
HCV[1] |
| In Vitro | Pibrentasvir inhibits HCV genotype 1a-H77, 1b-Con1, and 2a-JFH-1 subgenomic replicons with 50% effective concentrations (EC50s) of 1.8, 4.3, and 5.0 pM, respectively. The antiviral activity of Pibrentasvir is attenuated 35- to 47-fold in the presence of 40% human plasma through sequestration of compound due to plasma protein binding. Pibrentasvir retains full activity against all of the genotype 1a and 1b single-position NS5A substitutions tested, except Y93H and Y93N in genotype 1a, which confers a ≤7-fold increase in EC50 to Pibrentasvir[1]. |
| Cell Assay | The inhibitory effect of Pibrentasvir on HCV replication in replicon cells is determined in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal bovine serum with or without 40% human plasma. The cells are incubated with Pibrentasvir for 3 days and are subsequently lysed and processed according to the manufacturer's instructions to measure luciferase reporter activity using a Victor II luminometer. The 50% effective concentration (EC50) value is calculated using nonlinear regression curve fitting to the four-parameter logistic equation in software[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C57H65F5N10O8 |
| Molecular Weight | 1113.18000 |
| Exact Mass | 1112.49000 |
| PSA | 199.58000 |
| LogP | 8.71 |
| Index of Refraction | 1.614 |
| Storage condition | 2-8℃ |