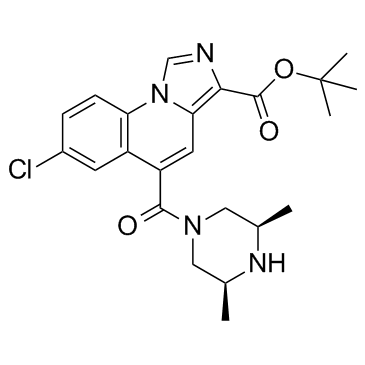
170568-47-5
| Name | tert-Butyl 7-chloro-5- [(cis-3,5-dimethylpiperazino)carbonyl]imidazo-[1,5-a]quinoline-3-carboxylate |
|---|---|
| Synonyms |
tert-butyl 7-chloro-5-[(cis-3,5-dimethylpiperazino)carbonyl]imidazo[1,5-a]quinoline-3-carboxylate
U-101017 |
| Description | U-101017 is a partial agonist of benzodiazepine receptor and GABAA receptor, with anxiolytic effects. |
|---|---|
| Related Catalog | |
| In Vitro | PNU-101017 potentiates GABA-stimulated Cl- currents at low concentrations (<1 μM)[1]. U-101017 concentration-dependently inhibits the binding of [3H]FNZ to the membrane preparation of rat cerebral cortex in vitro with Ki of 3.37±0.22 nM[2]. |
| In Vivo | Pre-ischemic treatment with either PNU-101017 significantly protects the CA1 neuronal population, and PNU-101017 reduces the loss to 50%. Delaying PNU-101017 administration until immediately after reperfusion does not reduce the neuroprotective activity[1]. U-101017 (30 μmol/kg, p.o.) time-dependently blocks [3H]FNZ binding to the mouse cerebral cortex. U-101017 dose-dependently decreases the levels of cGMP with ED50s of 260.0 (163-425) and 0.37 (0.12-1.04) in nonstressed and foot shock-stressed mice, respectively. Flumazenil, an antagonist of GABAA receptors, has no significant effect on cGMP in nonstressed mice, but pretreatment with flumazenil significantly blocks U-101017 (10 μmol/kg, p.o.)-induced reductions in cGMP. In stressed mice, flumazenil is ineffective in altering cerebellar cGMP, but pretreatment with these doses of flumazenil significantly (p < 0.01) blocks U-101017-induced attenuation of stress-induced elevations in cGMP[2]. |
| Animal Admin | Three groups of gerbils (N=9-11/group) are treated i.p. with either vehicle (0.05 N HCl), PNU-101017 (30 mg/kg) or diazepam (10 mg/kg) 30 min prior to ischemia and again 2 h after reperfusion. Two other groups receive PNU-101017 or diazepam immediately after reperfusion and again 2 h later. The tested doses of PNU-101017 and diazepam are selected from past studies demonstrating their neuroprotective efficacy in the gerbil forebrain ischemia model. The administration of the second dose at 2 h after reperfusion is consistent with previous dosing with other effective compounds tested in the gerbil. The 0.05 N HCl vehicle has been employed for i.p. dosing with other test compounds and is devoid of toxicity or acute distress production. |
| References |
| Molecular Formula | C23H27ClN4O3 |
|---|---|
| Molecular Weight | 442.93800 |
| Exact Mass | 442.17700 |
| PSA | 75.94000 |
| LogP | 4.18530 |
| Storage condition | 2-8℃ |