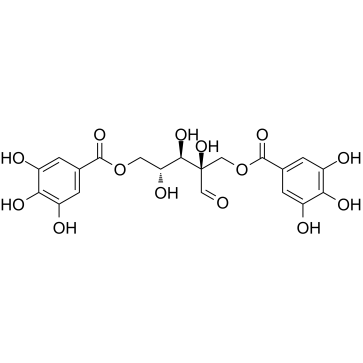
469-32-9
| Name | hamamelitannin |
|---|---|
| Synonyms |
HAMAMELOFURANOSE 2',5-DIGALLATE
2',5-DigalloylhaMaMelofuranose 2,3-Digalloylglucose [(2R,3R,4R)-3,4,5-Trihydroxytetrahydrofuran-2,4-diyl]bis(methylen)bis(3,4,5-trihydroxybenzoat) 2-C-[[(3,4,5-Trihydroxybenzoyl)oxy]methyl]-D-ribofuranose 5-(3,4,5-trihydroxybenzoate) Bis(3,4,5-trihydroxybenzoate) de [(2R,3R,4R)-3,4,5-trihydroxytétrahydrofurane-2,4-diyl]bis(méthylène) 2',5-digalloylhamamelose hamamelitanin Hamamelitannin [(2R,3R,4R)-3,4,5-Trihydroxytetrahydrofuran-2,4-diyl]bis(methylene) bis(3,4,5-trihydroxybenzoate) O5-Galloyl-2-galloyloxymethyl-D-ribose 5-O-(3,4,5-Trihydroxybenzoyl)-2-C-{[(3,4,5-trihydroxybenzoyl)oxy]methyl}-D-ribofuranose D-Ribofuranose, 2-C-[[(3,4,5-trihydroxybenzoyl)oxy]methyl]-, 5-(3,4,5-trihydroxybenzoate) 2-C-(Hydroxymethyl)-D-ribofuranose 2',5-Digallate |
| Description | Hamamelitannin, a polyphenol extracted from the bark of Hamamelis virginiana, is a quorum-sensing (QS) inhibitor. Hamamelitannin increases antibiotic susceptibility of staphylococcus aureus biofilms by affecting peptidoglycan biosynthesis and eDNA release[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Hamamelitannin displays specific cytotoxic activity against colon cancer cells[3]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 941.7±65.0 °C at 760 mmHg |
| Melting Point | 145-147ºC |
| Molecular Formula | C20H20O14 |
| Molecular Weight | 484.364 |
| Flash Point | 329.0±27.8 °C |
| Exact Mass | 484.085297 |
| PSA | 243.90000 |
| LogP | 2.91 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.746 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |