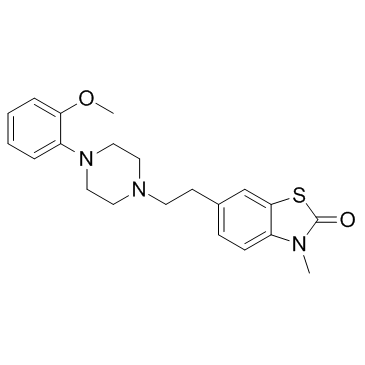
142477-34-7
| Name | 5-HT1A modulator 1 |
|---|
| Description | 5-HT1A modulator 1 displays very high affinities for the 5HT1A, adrenergic α1 and dopamine D2 receptor with IC50s of 2 ±0.3 nM, 10 ± 3 nM and 40 ±9 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
5-HT1A Receptor:2 nM (IC50) 5-HT1B Receptor:300 nM (IC50) 5-HT2A Receptor:500 nM (IC50) 5-HT2C Receptor:4000 nM (IC50) α1 receptor:10 nM (IC50) D2 receptor:40 nM (IC50) |
| In Vitro | 5-HT1A modulator 1 (Compound 24) also displays affinities for the 5HT1B, 5-HT2A and 5-HT2C receptor with IC50s of 300±55 nM, 500±75 nM, and 4000±440 nM, respectively[1]. |
| In Vivo | 5-HT1A modulator 1 (Compound 24) shows clear antagonist action at 5HT2A receptor subtype in mice. The antagonism is nearly complete at the dose of 1 mg/kg ip for 5-HT1A modulator 1 (94% of antagonism, p<0.01). 5-HT1A modulator 1 completely blocks the stereotypies and the climbing at the dose of 1 mg/kg ip (100% of antagonism). 5-HT1A modulator 1 is also tested in rats, using the same paradigm. After oral administration, 5-HT1A modulator 1 significantly (p<0.05) reduces the hyperactivity by 50% at the doses of 2 and 4 mg/kg po, respectively 63% and 58% of antagonism for 5-HT1A modulator 1; the antagonism is complete (103% and 108%) at the respective doses of 8 and 16 mg/kg po for 5-HT1A modulator 1 (p<0.01)[1]. |
| Kinase Assay | Binding is determined using membranes prepared from bovine hippocampus. The receptor is labeled with 0.5 nM [3H]-8-hydroxydipropylaminotetralin (8-OH-DPAT) by incubation at 25°C for 30 min with 11 concentrations of the test compounds (1-105 nM). Nonspecific binding is determined using 10-5 Mbuspirone. Competition experiments are analyzed using the iterative nonlinear least-squares curve-fitting program Inplot 4, graphpad; IC50 values are calculated using the Cheng-Prusoff equation[1]. |
| Animal Admin | Rats[1] Wistar rats (n=6) are used. 5-HT1A modulator 1 is tested at pharmacological doses (1 and 2 mg/kg ip, respectively) and at high doses (32 and 64 mg/kg ip) in rats. The intensity of forepaw treading is expressed as percentage of the maximal possible score. The 5HT1A agonist 8-OH-DPAT induces forepaw treading and is used as a reference compound. Mice[1] Swiss mice are injected with the test compound (e.g., 5-HT1A modulator 1, 0.25 and 1 mg/kg ip) before an injection of 5HTP (400 mg/kg ip). The number of head twiches occurring in a 10 min period starting 10 min after the injection of 5HTP is counted. Cyproheptadine is used as reference compound. |
| References |
| Molecular Formula | C21H25N3O2S |
|---|---|
| Molecular Weight | 383.51 |
| Storage condition | 2-8℃ |