1794817-45-0
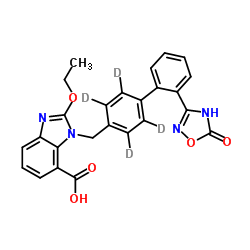
| Name | 2-Ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)(2,3,5,6-2H4)-4-biphenylyl]methyl}-1H-benzimidazole-7-carboxylic acid |
|---|---|
| Synonyms |
1H-Benzimidazole-7-carboxylic acid, 1-[[2'-(4,5-dihydro-5-oxo-1,2,4-oxadiazol-3-yl)[1,1'-biphenyl]-4-yl-2,3,5,6-d4]methyl]-2-ethoxy-
2-Ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)(2,3,5,6-2H4)-4-biphenylyl]methyl}-1H-benzimidazole-7-carboxylic acid |
| Description | Azilsartan-d4 is the deuterium labeled Azilsartan[1]. Azilsartan is an orally active, potent, selective and specific angiotensin II type 1 receptor (AT1) antagonist. Azilsartan induces ROS formation and apoptosis in HepG2 cells. Azilsartan shows neuroprotective and anticancer activity. Azilsartan can be used for hypertension and stroke research[2][3][4][5][6]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C25H16D4N4O5 |
| Molecular Weight | 460.475 |
| Exact Mass | 460.168488 |
| LogP | 4.21 |
| Index of Refraction | 1.695 |
| Storage condition | 2-8°C |