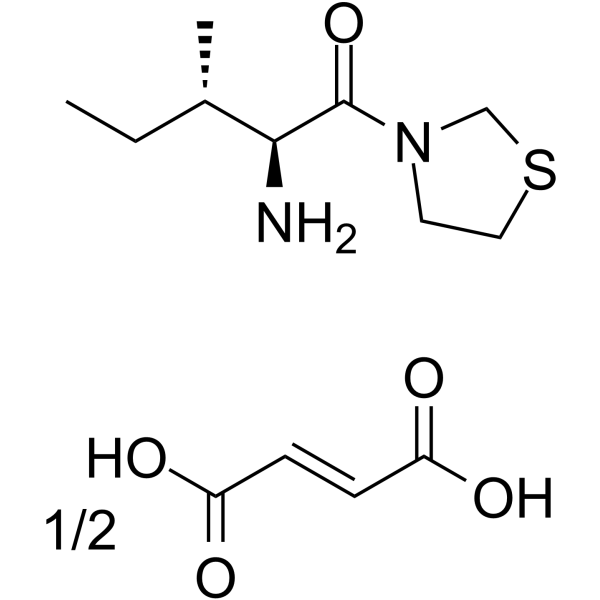
251572-86-8
| Name | Thiazolidine,3-[(2S,3S)-2-aMino-3-Methyl-1-oxopentyl]-, (2E)-2-butenedioate (2:1) |
|---|---|
| Synonyms | NONE |
| Description | P32/98 hemifumarateis a potent inhibitor of dipeptidyl peptidase IV with a Ki value of 130 nM. P32/98 hemifumarate improves glucose tolerance, insulin sensitivity and β-cell responsiveness in fatty Zucker rat model[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
DPP-IV:130 nM (Ki) |
| In Vitro | GLP-1 acts function of stimulation of glucose dependent insulin secretion and induction of satiety feelings, and DPPIV is the major renal catabolic pathway for GLP-1 in vivo[2]. P32/98 hemifumarate, together with 200 pM GLP-1, (10 μM; 3 h) shows no significant inhibition of sodium re-absorption in porcine proximal tubular cells[2]. P32/98 hemifumarate (10 μM; 96 h) does not influence the mRNA expression of GLP-1R, DPPIV, Na+/H+ exchanger isoform 3 (NHE3), sodium-dependent glucose transporter slc5a1, slc5a2 (SGLT1, 2)[2]. Cell Cytotoxicity Assay[2] Cell Line: Porcine proximal tubular cells Concentration: 10 μM Incubation Time: 96 hours Result: Showed no toxic. |
| In Vivo | P32/98 hemifumarate (25 mg/kg; i.g.; once daily) long-time treatment significantly improves the glucose tolerance in Zucker diabetic fatty rats, a model of IGT (impaired glucose tolerance)[3]. Animal Model: Zucker diabetic fatty rat[3] Dosage: 25 mg/kg Administration: Oral gavage; once daily Result: Significantly improved the glucose tolerance in Zucker diabetic fatty rats. |
| References |
| Molecular Formula | C22H40N4O6S2 |
|---|---|
| Molecular Weight | 520.706 |
| Exact Mass | 520.238953 |