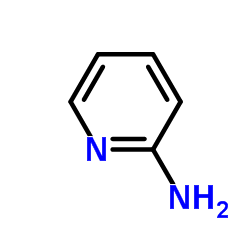
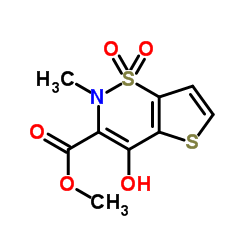
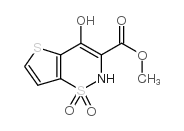
59804-37-4
| Name | tenoxicam |
|---|---|
| Synonyms |
Apo-Tenoxicam
4-hydroxy-2-methyl-N-(pyridin-2-yl)-2H-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide Mobiflex Tenoxicam Tenoxicamum Octiveran 4-Hydroxy-2-methyl-N-2-pyridinyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxamide1,1-Dioxide 4-hydroxy-2-methyl-N-(pyridin-2-yl)-2H-thieno2,3-e1,2thiazine-3-carboxamide 1,1-dioxide 4-hydroxy-2-methyl-1,1-dioxo-N-pyridin-2-ylthieno[2,3-e]thiazine-3-carboxamide 4-Hydroxy-2-methyl-N-(2-pyridinyl)-2H-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide 2H-thieno2,3-e-1,2-thiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide Tilcotil 2H-Thieno[2,3-e]-1,2-thiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide Liman Novo-Tenoxicam |
| Description | Tenoxicam, an antiinflammatory agent with analgesic and antipyretic properties.Target: COXTenoxicam is a non-steroidal anti-inflammatory drug (NSAID). Tenoxicam-treated patients had significant decrease in nitrite levels (p = 0.036) and XO activity (p = 0.01), but their SOD, GSH-Px enzyme activities, and MDA levels were unchanged from baseline. Tenoxicam may have antioxidant effects, and it may reduce nitrite levels, indicating an alteration of NO pathways [1]. Tenoxicam was administered intraperitoneally immediately after BCAO. Histological analyses show that ischemia produced significant striatal as well as hippocampal lesions which were reversed by the Tenoxicam treatment. Tenoxicam also significantly reduced, to control levels, the increased myeloperoxidase activity in hippocampus homogenates observed after ischemia [2]. |
|---|---|
| Related Catalog | |
| Target |
COX-1 COX-2 |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Melting Point | 209-2130ºC |
| Molecular Formula | C13H11N3O4S2 |
| Molecular Weight | 337.374 |
| Exact Mass | 337.019104 |
| PSA | 136.22000 |
| LogP | 1.52 |
| Index of Refraction | 1.741 |
| Storage condition | -20°C Freezer |
| Water Solubility | Practically insoluble in water, sparingly soluble in methylene chloride, very slightly soluble in anhydrous ethanol. It dissolves in solutions of acids and alkalis. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H311-H331 |
| Precautionary Statements | P261-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R23/24/25 |
| Safety Phrases | S36/37/39-S45 |
| RIDADR | UN 2811 |
| WGK Germany | 2 |
| RTECS | XJ9095060 |
| HS Code | 2934999090 |
|
~75% 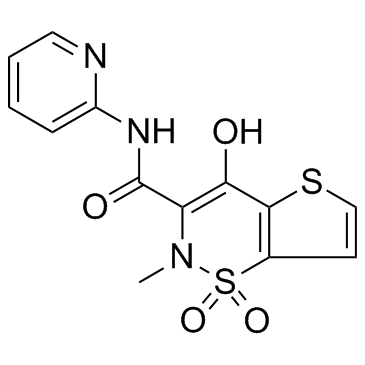
59804-37-4 |
| Literature: Binder, Dieter; Hromatka, Otto; Geissler, Franz; Schmied, Karl; Noe, Christian R.; et al. Journal of Medicinal Chemistry, 1987 , vol. 30, # 4 p. 678 - 682 |
|
~% 
59804-37-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 678 - 682 |
|
~% 
59804-37-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 678 - 682 |
|
~% 
59804-37-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 678 - 682 |
|
~% 
59804-37-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 678 - 682 |
|
~% 
59804-37-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 678 - 682 |
|
~% 
59804-37-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 678 - 682 |
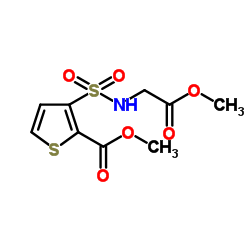
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |