60-56-0
| Name | methimazole |
|---|---|
| Synonyms |
1-methyl-1H-imidazole-2(3H)-thione
Methimazole 2H-Imidazole-2-thione, 1,3-dihydro-1-methyl- 3-methyl-imidazole-2-thione methyl-2-mercaptoimidazole 1-methyl-1H-imidazole-2-thiol Thimazol Merkastan Frentirox 2-mercapto-1-methyl-imidazole 2-Mercapto-1-methylimidazole Metazolo Thymidazole thioimidazole 1-Methyl-2-mercapto-1H-imidazole mercaptomethylimidazole Basolan 1-Methyl-1,3-dihydro-2H-imidazole-2-thione 1-Methyl-4-imidazoline-2-thione Tapazole Methiamazole Methimazol Metizol Tapuzole 1-methylimidazole-2-thione METAZOLE thiamazole 1-methyl-2-mercapto-imidazole N-METHYLIMIDAZOLETHIOL Mercazole Favistan 2-Mercapto-1-methyl-1H-imidazole MFCD00179321 EINECS 200-482-4 |
| Description | Methimazole(Tapazole, Northyx) is an antithyroid medicine.Target: OthersMethimazole is an antithyroid drug, and part of the thioamide group. Like its counterpart propylthiouracil, a major side effect of treatment is agranulocytosis. Methimazole is a drug used to treat hyperthyroidism, a condition that occurs when the thyroid gland begins to produce an excess of thyroid hormone. The drug may also be taken before thyroid surgery to lower thyroid hormone levels and minimize the effects of thyroid manipulation. Additionally, Methimazole is used in the veterinary setting to treat hyperthyroidism in cats.Methimazole inhibits the enzyme thyroperoxidase, which normally acts in thyroid hormone synthesis by oxidizing the anion iodide (I-) to iodine (I0), facilitating iodine's addition to tyrosine residues on the hormone precursor thyroglobulin, a necessary step in the synthesis of triiodothyronine (T3) and thyroxine. It does not inhibit the action of the sodium-dependent iodide transporter located on follicular cells' basolateral membranes. Inhibition of this step requires competitive inhibitors such as perchlorate and thiocyanate.It acts at CXCL10. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 280.0±9.0 °C at 760 mmHg |
| Melting Point | 144-147 °C(lit.) |
| Molecular Formula | C4H6N2S |
| Molecular Weight | 114.169 |
| Flash Point | 123.1±18.7 °C |
| Exact Mass | 114.025169 |
| PSA | 56.62000 |
| LogP | -0.34 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.633 |
| Storage condition | Refrigerator |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H361 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R43;R62;R63 |
| Safety Phrases | S26-S27-S36-S45-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NI8615000 |
| HS Code | 2933290090 |
|
~% 
60-56-0 |
| Literature: Heterocycles, , vol. 17, p. 125 - 138 |
|
~% 
60-56-0 |
| Literature: European Journal of Inorganic Chemistry, , # 13 p. 2502 - 2511 |
|
~% 
60-56-0 |
| Literature: Journal of the American Chemical Society, , vol. 71, p. 4000 |
|
~% 
60-56-0 |
| Literature: Journal of the Chemical Society, , p. 1806,1809 |
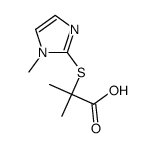
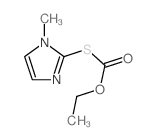
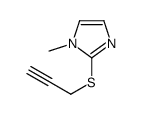
| Precursor 5 | |
|---|---|
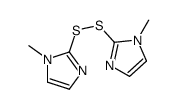
| DownStream 10 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


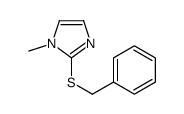
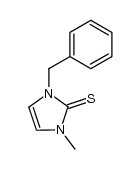
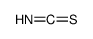
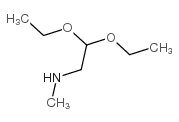

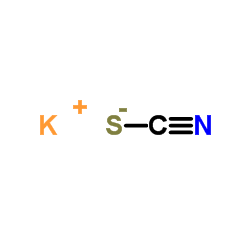

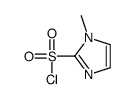
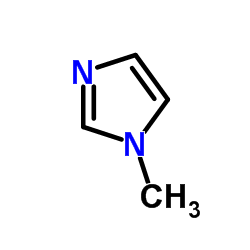
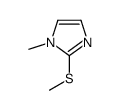
![[(1-METHYL-1H-IMIDAZOL-2-YL)SULFANYL]ACETIC ACID structure](https://image.chemsrc.com/caspic/240/71370-42-8.png)