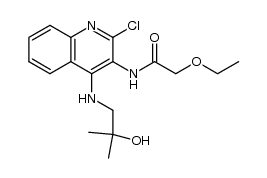
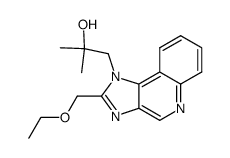
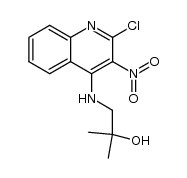
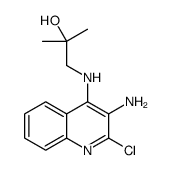
144875-48-9
| Name | resiquimod |
|---|---|
| Synonyms |
1-[4-Amino-2-(ethoxymethyl)-1H-imidazo[4,5-c]quinolin-1-yl]-2-methyl-2-propanol
Resiquimod 1-[4-amino-2-(ethoxymethyl)imidazo[4,5-c]quinolin-1-yl]-2-methylpropan-2-ol 1-(4-Amino-2-(ethoxymethyl)-1H-imidazo[4,5-c]quinolin-1-yl)-2-methylpropan-2-ol 4-amino-2-(ethoxymethyl)-a,a-dimethyl-1h-imidazo[4,5-c]quinoline-1-ethanol 1-[4-Amino-2-(ethoxymethyl)-1H-imidazo[4,5-c]quinolin-1-yl]-2-methylpropan-2-ol R848 |
| Description | Resiquimod is a Toll-like receptor 7 and 8 (TLR7/TLR8) agonist that induces the upregulation of cytokines such as TNF-α, IL-6 and IFN-α. |
|---|---|
| Related Catalog | |
| In Vitro | Resiquimod (R-848) induces both hapten- and allergen-specific circulating T cells, including TH2 effectors, to produce IFN-γ and even to lose the ability to produce IL-4[2]. Resiquimod (R848) enhances PBL proliferation in a dose-dependent manner, and increases the number of BrdU-positive cells in BrdU incorporation assay. Cells treated with R848 exhibits significantly increased (3.5-fold) luciferase (a reporter of NF-κB activity) activity[3]. |
| In Vivo | Resiquimod (R-848) (50 μg/bird, i.m. route) significantly up-regulates the expression of IFN-α, IFN-β, IFN-γ, IL-1β, IL-4, iNOS and MHC-II genes in SPF chicken[1]. |
| Kinase Assay | For luciferase assay, FG-9307 cells are transfected with the firefly NF-κB-specific luciferase reporter vector pNFκB-Met-Luc2. Transfection efficiency is monitored by co-transfection with the pSEAP2 control vector, which constitutively expresses the human secreted enhanced alkaline phosphatase (SEAP). Then the cells are treated with Resiquimod (R848, 1 µg/mL), CQ (10 µM), CQ plus R848 or PBS and incubated at 22°C for 24 h. The culture medium of the transfectants is then analyzed for luciferase activity and SEAP activity using Luciferase Assay Kit and the Great EscAPe™ SEAP Chemiluminescence Detection Kit, respectively. The assay is performed three times. |
| Cell Assay | For inhibition of lysosomal acidification, cells are incubated with 10 µM CQ for 1 h before Resiquimod (R848) treatment. After treatment, 20 µL of 5 mg/mL MTT is added to the plate. The plate is incubated at 22°C for 4 h, and 200 µL dimethyl sulfoxide is added to the plate to dissolve the reduced formazan. The plate is then read at 490 nm with a microplate reader. To determine the effect of Myd88 inhibition on R848-induced cell proliferation, the Myd88 inhibitor Pepinh-MYD and the control peptide Pepinh-Control are added to PBL at the concentration of 50 µM, and the plate is incubated at 22°C for 6 h. After incubation, the cells are treated with R848 and subjected to MTT assay as above. To determine the effect of NF-κB inactivation on R848-induced cell proliferation, BAY-11-7082, an irreversible inhibitor of IκB-α phosphorylation, is added to the cells at the concentration of 1 µM, and the plate is incubated at 22°C for 1 h. After incubation, the cells are treated with R848 and subjected to MTT assay as earlier. All experiments are performed three times. |
| Animal Admin | A total of 40 SPF chickens of two-week old are allotted to one of the following four experimental groups (n=10/group): Group A: PBS control; Group B: inactivated NDV vaccine; Group C: commercial oil adjuvanted inactivated NDV vaccine prepared from lentogenic strain and Group D: combination of inactivated NDV vaccine and R-848 (50 μg/bird). Vaccine or PBS is administered by intramuscular route in the thigh muscle. A booster dose is given 14-day post immunization (d.p.i). Two weeks post-booster, experimental SPF birds are challenged with velogenic strain of NDV (105 ELD50 per bird) intramuscularly. Clinical signs and mortality are observed daily till 14 day post-challenge (d.p.c). Cloacal swabs (n=6/group) are collected from the birds on day 0, 4, 7 and 14 post-challenge and inoculated into 10-day old embryonated chicken eggs (n=3 eggs/sample) through intra-allantoic route. Three day post-inoculation, the allantoic fluid is checked for the NDV growth by spot haemagglutination using 10% chicken RBC. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 553.6±50.0 °C at 760 mmHg |
| Melting Point | 193 °C |
| Molecular Formula | C17H22N4O2 |
| Molecular Weight | 314.382 |
| Flash Point | 288.6±30.1 °C |
| Exact Mass | 314.174286 |
| PSA | 86.19000 |
| LogP | 2.15 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.635 |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Resiquimod : SML0196 REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later
registration deadline. CAS-No.: 144875-48-9 SECTION 2: Hazards identification Classification of the substance or mixture Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. Not a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC. Label elements The product does not need to be labelled in accordance with EC directives or respective national laws. Other hazards - none SECTION 3: Composition/information on ingredients Substances Synonyms: 4-Amino-2-(ethoxymethyl)-alpha,alpha-dimethyl-1H-imidazo(4,5- c)quinoline-1-ethanol R-848 Formula: C17H22N4O2 Molecular Weight: 314,38 g/mol CAS-No.: 144875-48-9 No components need to be disclosed according to the applicable regulations. SECTION 4: First aid measures Description of first aid measures If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. In case of skin contact Wash off with soap and plenty of water. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture no data available Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Avoid dust formation. Avoid breathing vapours, mist or gas. For personal protection see section 8. Environmental precautions Do not let product enter drains. Methods and materials for containment and cleaning up Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Provide appropriate exhaust ventilation at places where dust is formed. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: -20 °C Hygroscopic. Store with desiccant. Specific end use(s) A part from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls General industrial hygiene practice. Personal protective equipment Eye/face protection Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. Body Protection Choose body protection in relation to its type, to the concentration and amount of dangerous substances, and to the specific work-place., The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Respiratory protection is not required. Where protection from nuisance levels of dusts are desired, use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Do not let product enter drains. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: powder b) Odourno data available c) Odour Thresholdno data available d) pH7,2 e) Melting point/freezing166 °C point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- log Pow: 2,109 octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity no data available Biochemical:Metabolism (intermediary): Effect on inflammation or mediation of inflammation. Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: NJ5911320 To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15: Regulatory information This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006. Safety, health and environmental regulations/legislation specific for the substance or mixture no data available Chemical Safety Assessment SECTION 16 - ADDITIONAL INFORMATION N/A |
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | NJ5911320 |
| HS Code | 2933990090 |
|
~% 
144875-48-9 |
| Literature: US6348462 B1, ; Page column 33, 34 ; |
|
~% 
144875-48-9 |
| Literature: ASCEND BIOPHARMACEUTICALS PTY LTD; PIETERSZ, Geoffrey, Alan Patent: WO2013/67597 A1, 2013 ; Location in patent: Page/Page column 80 ; |
|
~% 
144875-48-9 |
| Literature: US6465654 B2, ; |
|
~% 
144875-48-9 |
| Literature: WO2013/67597 A1, ; |
|
~% 
144875-48-9 |
| Literature: WO2013/67597 A1, ; |
|
~% 
144875-48-9 |
| Literature: WO2013/67597 A1, ; |
|
~% 
144875-48-9 |
| Literature: WO2013/67597 A1, ; |
|
~% 
144875-48-9 |
| Literature: WO2013/67597 A1, ; |
| Precursor 7 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |