1842399-68-1
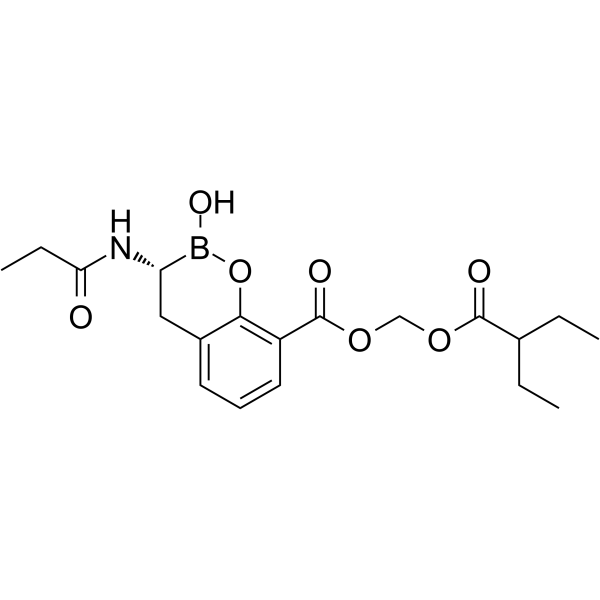
| Name | [(2-Ethylbutanoyl)oxy]methyl (3R)-2-hydroxy-3-(propionylamino)-3,4-dihydro-2H-1,2-benzoxaborinine-8-carboxylate |
|---|---|
| Synonyms |
2H-1,2-Benzoxaborin-8-carboxylic acid, 3,4-dihydro-2-hydroxy-3-[(1-oxopropyl)amino]-, (2-ethyl-1-oxobutoxy)methyl ester, (3R)-
[(2-Ethylbutanoyl)oxy]methyl (3R)-2-hydroxy-3-(propionylamino)-3,4-dihydro-2H-1,2-benzoxaborinine-8-carboxylate |
| Description | Ledaborbactam etzadroxil (VNRX-7145) is an orally active Ambler class A, C, and D β-lactamase enzymes inhibitor[1]. |
|---|---|
| Related Catalog | |
| Target |
β-Lactamase[1] |
| In Vivo | Upon dosing the Ledaborbactam etzadroxil (VNRX-7145) in mice, dogs, and monkeys, Ledaborbactam etzadroxil (5-10 mg/kg) demonstrates the most consistent oral bioavailability across species (F = 61-82%)[1]. In intestinal S9, Ledaborbactam etzadroxil is rapidly cleaved with short half-lives in all species with the exception of beagle dogs. The half-life in human plasma is short at about 11 min, which is closer to what was observed in the rodent species compared to the longer half-lives in dogs (43.9 min) and monkeys (22.0 min)[1]. In vivo efficacy is demonstrated in a lethal murine septicemia model by dosing Ledaborbactam etzadroxil (orally) with Ceftibuten. The ED50 value of 12.9 mg/kg[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Molecular Formula | C19H26BNO7 |
| Molecular Weight | 391.22 |
| Exact Mass | 391.180237 |
| Index of Refraction | 1.531 |