480-41-1
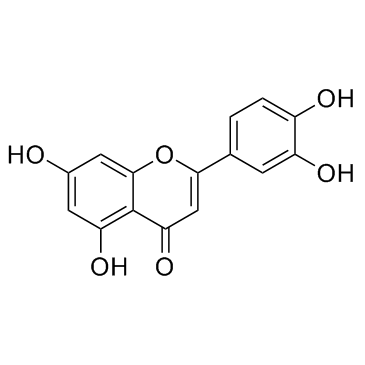
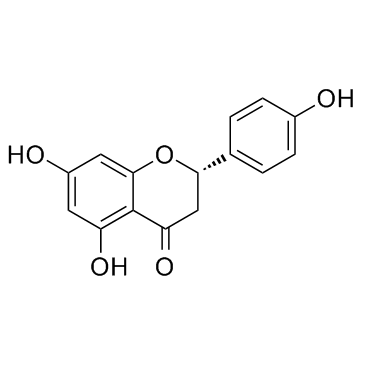
| Name | (S)-naringenin |
|---|---|
| Synonyms |
4′,5,7-Trihydroxyflavanone
4’,5,7-Trihydroxyflavanone Naringenin 4',5,7-trihydroxyflavanone (S)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one (±)-Naringenin Salipurol naringenine 4' 5 7-trihydroxyflavanone 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (S)- Derivative of (S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one 4‘,5,7-Trihydroxyflavanone (2S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one pelargidanon 1602 5,7,4'-trihydroxyflavanone 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-, (2S)- rac-naringenin Salipurpol MFCD00006844 Naringetol EINECS 266-769-1 |
| Description | Naringenin is the predominant flavanone in grapefruit; displays strong anti-inflammatory and antioxidant activities. |
|---|---|
| Related Catalog | |
| In Vitro | Naringenin is shown to inhibit the proliferation of HepG2 cells resulted partly from an accumulation of cells in the G0/G1 and G2/M phase of the cell cycle. Naringenin has been shown to induce apoptosis as evidenced by nuclei damage and increased proportion of apoptotic cells. Naringenin triggers the mitochondrial-mediated apoptosis pathway as shown by an increased ratio of Bax/Bcl-2, subsequent release of cytochrome C, and sequential activation of caspase-3[1]. Naringenin exposure significantly reduces the cell viability of A431 cells with a concomitant increase in nuclear condensation and DNA fragmentation in a dose dependent manner. Cell cycle study shows that naringenin induced cell cycle arrest in G0/G1 phase of cell cycle and caspase-3 analysis reveal a dose dependent increment in caspase-3 activity which leads to cell apoptosis[2]. |
| In Vivo | Naringenin supplementation causes a significant reduction in the amount of total triglyceride and cholesterol in plasma and liver. In addition, naringenin supplementation lowers adiposity and triglyceride contents in parametrial adipose tissue. Naringenin-fed animals show a significant increase in PPARα protein expression in the liver. The expression of CPT-1 and UCP2, known to be regulated by PPARα, is markedly enhanced by naringenin treatment[3]. Naringenin increases hepatic fatty acid oxidation through a PPARγ coactivator 1α/PPARα-mediated transcription program. It prevents sterol regulatory element-binding protein 1c–mediated lipogenesis in both liver and muscle by reducing fasting hyperinsulinemia. Naringenin decreases hepatic cholesterol and cholesterol ester synthesis[4]. Naringenin inhibits TNF-α-induced VSMC proliferation and migration in a dose-dependent manner. Mechanistic study demonstrates that naringenin prevents ERK/MAPK and Akt phosphorylation while left p38 MAPK and JNK unchanged. Naringenin also blocks the increase of ROS generation induced by TNF-α[5]. |
| Cell Assay | Naringenin is dissolved in DMSO and diluted in cell culture medium. The cells are rinsed with PBS and grown in a medium containing various concentrations of naringenin (50, 100, 150, 200, 250, 300 μM). The solvent DMSO treated cells are served as control. After 24 hrs of treatment, the medium is removed and replaced by another medium containing MTT. Cell viability is measured using the MTT assay[1]. |
| Animal Admin | Rats: Semi-purified, powdered diets are prepared for concentrations of naringenin: 0, 0.003, 0.006, and 0.012% of diet. After 7 days of acclimatization, rats are assigned to one of four groups, with six animals per group, and fed semi-purified experimental diets for 6 weeks. The experimental diets contain 16% fat, 45.5% sucrose, and different naringenin concentration (0, 0.003, 0.006, or 0.012%) (Table 1). Rats have ad libitum access to food and water during the study period. Food intake and body weight are measured throughout the experiment[3]. Mouse: Eight- to 12-week-old mice are fed ad libitum a rodent standard diet or a high-fat diet containing 42% of calories from fat plus cholesterol (0.05% wt/wt). Naringenin is added to the Western diet at 1 or 3% (wt/wt). Ldlr−/− mice are fed for 4 weeks and C57BL/6J mice for 30 weeks. Food intake is measured daily, and body weight is measured biweekly. Mice are fasted for 6 h before intervention[4]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 577.5±50.0 °C at 760 mmHg |
| Melting Point | 247-250 °C(lit.) |
| Molecular Formula | C15H12O5 |
| Molecular Weight | 272.253 |
| Flash Point | 224.7±23.6 °C |
| Exact Mass | 272.068481 |
| PSA | 86.99000 |
| LogP | 3.19 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.693 |
| Storage condition | -20°C Freezer |
Synonym:Naringenin Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 36/37/38 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin. Potential Health Effects Eye: Causes eye irritation. May cause chemical conjunctivitis. Skin: Causes skin irritation. Ingestion: May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated. Inhalation: Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema. Chronic: Effects may be delayed. Section 4 - FIRST AID MEASURES Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Ingestion: Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. Notes to Physician: Treat symptomatically and supportively. Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution. Extinguishing Media: Use foam, dry chemical, or carbon dioxide. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation. Section 7 - HANDLING and STORAGE Handling: Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse. Storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 480-41-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Powder Color: beige - brown Odor: Not available. pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: 247 - 250 deg C Autoignition Temperature: Not available. Flash Point: Not available. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: Specific Gravity/Density: Molecular Formula: Molecular Weight: 272.26 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable at room temperature in closed containers under normal storage and handling conditions. Conditions to Avoid: Incompatible materials, dust generation, excess heat, strong oxidants. Incompatibilities with Other Materials: Oxidizing agents. Hazardous Decomposition Products: Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide. Hazardous Polymerization: Has not been reported Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 480-41-1: DJ2981530 LD50/LC50: Not available. Carcinogenicity: 4',5,7-Trihydroxyflavanone - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: Not regulated. Hazard Class: UN Number: Packing Group: IMO Shipping Name: Not regulated. Hazard Class: UN Number: Packing Group: RID/ADR Shipping Name: Not regulated. Hazard Class: UN Number: Packing group: Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: XI Risk Phrases: R 36/37/38 Irritating to eyes, respiratory system and skin. Safety Phrases: S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. S 37/39 Wear suitable gloves and eye/face protection. WGK (Water Danger/Protection) CAS# 480-41-1: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 480-41-1 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 480-41-1 is not listed on the TSCA inventory. It is for research and development use only. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Hazard Codes | Xn:Harmful |
|---|---|
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| WGK Germany | 3 |
| HS Code | 2932999099 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![4-[(3-Methyl-2-buten-1-yl)oxy]benzaldehyde structure](https://image.chemsrc.com/caspic/390/28090-12-2.png)
![1-[2-hydroxy-4,6-bis(methoxymethoxy)phenyl]ethanone structure](https://image.chemsrc.com/caspic/448/65490-09-7.png)