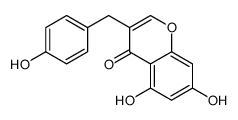
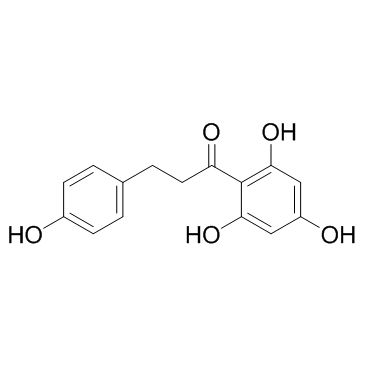
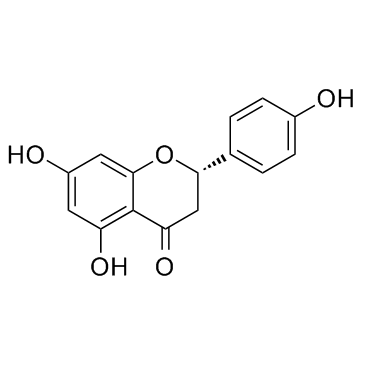
107585-77-3
| Name | 5,7-Dihydroxy-3-(4-hydroxybenzyl)-2,3-dihydro-4H-chromen-4-one |
|---|---|
| Synonyms |
4'-demethyl-5-O-methyl-dihydroeucomin
iristectorigenin-A Iristectorigenin B 4'-demethyl-3,9-dihydropunctatin 4H-1-Benzopyran-4-one, 2,3-dihydro-5,7-dihydroxy-3-[(4-hydroxyphenyl)methyl]- 5,7-Dihydroxy-3-(4-hydroxybenzyl)-2,3-dihydro-4H-chromen-4-one 4,4'-demethyl-3,9-dihydropuctatin 5,7,4'-Trihydroxy-6,3'-dimethoxyisoflavone |
| Description | 4'-Demethyl-3,9-dihydroeucomin (Compound 3) is a high isoflavonoid derived from the agave Ledebouria floribund[1]. |
|---|---|
| Related Catalog | |
| References |
[1]. Calvo MI. Homoisoflavanones from Ledebouria floribunda. Fitoterapia. 2009 Mar;80(2):96-101. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 574.8±39.0 °C at 760 mmHg |
| Molecular Formula | C16H14O5 |
| Molecular Weight | 286.279 |
| Flash Point | 220.0±20.6 °C |
| Exact Mass | 286.084137 |
| PSA | 86.99000 |
| LogP | 3.65 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.684 |
| Hazard Codes | Xi |
|---|
|
~% 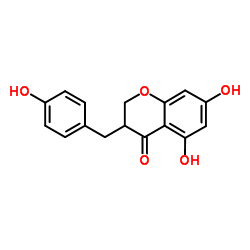
107585-77-3 |
| Literature: Sathyanarayana, S.; Krishnamurty, H. G. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1988 , vol. 27, # 1-12 p. 899 - 901 |
|
~% 
107585-77-3 |
| Literature: Sathyanarayana, S.; Krishnamurty, H. G. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1988 , vol. 27, # 1-12 p. 899 - 901 |
|
~% 
107585-77-3 |
| Literature: Sathyanarayana, S.; Krishnamurty, H. G. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1988 , vol. 27, # 1-12 p. 899 - 901 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |