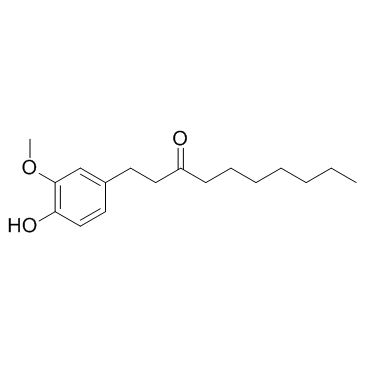
27113-22-0
| Name | 1-(4-hydroxy-3-methoxyphenyl)decan-3-one |
|---|---|
| Synonyms |
[6]-Paradol
7V2R DQ CO1 EINECS 248-228-1 1-(4-hydroxy-3-methoxy-phenyl)-decan-3-one 3-Decanone,1-(4-hydroxy-3-methoxyphenyl) 5-Paradol 1-(4-Hydroxy-3-methoxyphenyl)-3-decanone 1-(4-hydroxy-3-methoxyphenyl)decan-5-one 1-(4-Hydroxy-3-methoxy-phenyl)-decan-3-on 6-Paradol 3-Decanone, 1-(4-hydroxy-3-methoxyphenyl)- Paradol |
| Description | Paradol is a pungent phenolic substance found in ginger and other Zingiberaceae plants. Paradol is an effective inhibitor of tumor promotion in mouse skin carcinogenesis, binds to cyclooxygenase (COX)-2 active site. |
|---|---|
| Related Catalog | |
| Target |
COX-2 |
| In Vitro | Paradol ([6]-paradol) induces apoptosis in an oral squamous carcinoma cell line, KB, in a dose-dependent manner. Paradol induces apoptosis through a caspase-3-dependent mechanism[2]. |
| In Vivo | Administration of Paradol (6-paradol) (10 mg/kg) clearly reduces the number of Iba1-positive cells 1 and 3 days after the challenge. Moreover, Paradol dramatically reduces the number of Iba1-postive cells in periischemic regions even after 3 days following M/R challenge[3]. Paradol (6-paradol) exhibits the strongest anti-inflammatory effect of several paradol compounds in lipopolysaccharide-stimulated BV2 microglia derived from a mouse brain, including 2-, 4-, 6-, 8-, and 10-paradol. Furthermore, Paradol shows the strongest pungency of all of the known paradol analogues. Paradol also shows the highest contact time at the antiobesity site of action on the basis of the results shown for the absorption of the metabolites in this study[4]. |
| Cell Assay | KB, human oral epidermoid carcinoma cell lines (ATCC CCL-17) are plated at a density of 5×103 cells/200 μL/well into 96-well plate. After an overnight growth, the cells are treated with a series of paradol derivatives. All of the derivatives of paradol tested are dissolved in DMSO. The final concentration of DMSO in the culture medium is kept below 0.1% and the controls are treated with DMSO alone. Cell viability is assessed using MTT assay. In brief, after the cells are grown in the media in the absence or presence of the test compounds (e.g., Paradol, 10, 50, 100, 150, and 200 μM) for 48 h, they are then replaced to a 200 μL culture medium containing 0.5 mg/mL MTT for 3 h. The resulting MTT-formazan product is dissolved by an addition of the same volume of DMSO. The amount of formazan is determined by measuring the absorbance at 570 nm[2]. |
| Animal Admin | Mice[3] Male ICR mice (7 weeks old, 36±2 g) challenged with middle cerebral artery occlusion (MCAO)/reperfusion (M/R) are randomly divided into vehicle (10% Tween80)- or Paradol-administered groups (n=6~7 per group). Paradol dissolved in 10% Tween80 is orally administered (10 mg/kg) into mice at 1, 5, or 10 mg/kg immediately after reperfusion. Rats[4] Five-week-old Sprague-Dawley rats (male) are used. At 8 weeks of age, the rats are fasted for 14 h prior to the oral administration of olive oil (1 mL) containing zingerone or 6-, 8-, or 12-paradol (10 mg/kg). Three rats in each group are anesthetized with isoflurane, and samples (0.3 mL) of their blood are collected from their jugular vein using a heparinized needle and syringe at 0 (i.e., prior to the oral administration), 0.25, 0.5, 1, 3, 6, and 24 h after the oral administration of the olive oil containing test compounds. The AUC0-24h values determined using this time schedule are very similar compared with AUC0-24h that sampled the time points more minutely with other materials in our laboratory. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 406.5±30.0 °C at 760 mmHg |
| Molecular Formula | C17H26O3 |
| Molecular Weight | 278.387 |
| Flash Point | 140.0±18.1 °C |
| Exact Mass | 278.188202 |
| PSA | 46.53000 |
| LogP | 3.83 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.507 |
| Storage condition | -20°C |
| HS Code | 2914509090 |
|---|
|
~% 
27113-22-0 |
| Literature: Locksley,H.D. et al. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1972 , p. 3001 - 3006 |
|
~% 
27113-22-0 |
| Literature: Locksley,H.D. et al. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1972 , p. 3001 - 3006 |
|
~% 
27113-22-0 |
| Literature: Locksley,H.D. et al. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1972 , p. 3001 - 3006 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |