98079-51-7
| Name | lomefloxacin |
|---|---|
| Synonyms |
1-Ethyl-6,8-difluoro-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
LFLX Lomefloxacin Lomefloxacinum (±)-1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic Acid 1-Ethyl-6,8-difluoro-7-(3-methyl-1-piperazinyl)-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid Maxaquin 1-ethyl-6,8-difluoro-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid UNII:L6BR2WJD8V Lomefloxacino 3-Quinolinecarboxylic acid, 1-ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo- MFCD00242673 Lomefloxacine |
| Description | Lomefloxacin(SC47111A) is a fluoroquinolone antibiotic.Target: AntibacterialLomefloxacin is a bactericidal fluoroquinolone agent with activity against a wide range of gram-negative and gram-positive organisms. The bactericidal action of lomefloxacin results from interference with the activity of the bacterial enzymes DNA gyrase and topoisomerase IV, which are needed for the transcription and replication of bacterial DNA. DNA gyrase appears to be the primary quinolone target for gram-negative bacteria. Topoisomerase IV appears to be the preferential target in gram-positive organisms. Interference with these two topoisomerases results in strand breakage of the bacterial chromosome, supercoiling, and resealing. As a result DNA replication and transcription is inhibited [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 542.7±50.0 °C at 760 mmHg |
| Melting Point | 239-240ºC |
| Molecular Formula | C17H19F2N3O3 |
| Molecular Weight | 351.348 |
| Flash Point | 282.0±30.1 °C |
| Exact Mass | 351.139435 |
| PSA | 74.57000 |
| LogP | 1.71 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.566 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | Xn,Xi |
|---|---|
| Risk Phrases | 22-36/37/38 |
| Safety Phrases | S26-S36 |
| WGK Germany | 3 |
| RTECS | VB1997500 |
| HS Code | 2933990090 |
|
~93% 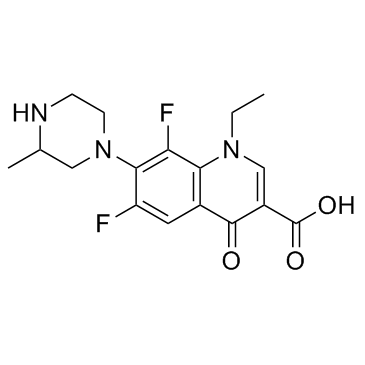
98079-51-7 |
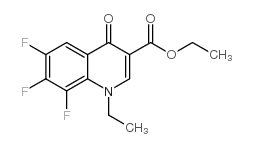
| Literature: BERTHON-CEDILLE, Laurence; Leguern, Marie-Emmanuelle; Renaud, Gilles; Tombret, Francis Patent: US2009/54643 A1, 2009 ; Location in patent: Page/Page column 6 ; |
|
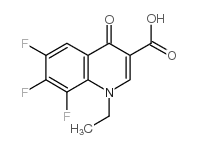
~85% 
98079-51-7 |
| Literature: Domagala; Bridges; Culbertson; Gambino; Hagen; Karrick; Porter; Sanchez; Sesnie; Spense; Szotek; Wemple Journal of Medicinal Chemistry, 1991 , vol. 34, # 3 p. 1142 - 1154 |

98079-51-7 ~% 
98079-51-7 |
| Literature: Journal of Chemical Crystallography, , vol. 39, # 5 p. 384 - 388 |
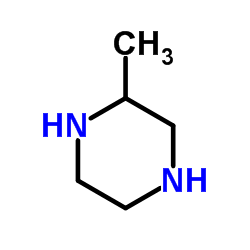
| Precursor 4 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |