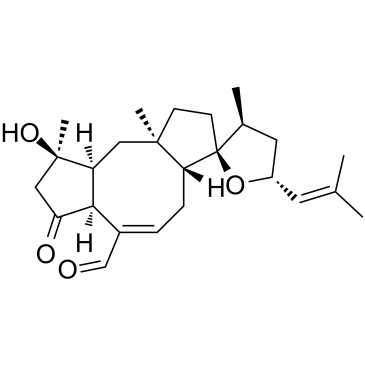
4611-05-6
| Name | ophiobolin A |
|---|---|
| Synonyms |
COCHLIOBOLIN A
(18R)-3-Hydroxy-5-oxo-14,18-epoxyophiobola-7,19-diene-25-al 18-epoxy-3-hydroxy-5-oxo-19-dien-25-a(18r)-ophiobola-14 Ophiobolin A cochliobolin ophiobolin Ophiobolin,Helminthosporium sp. orphiobolin A (7E,18R)-3-Hydroxy-5-oxo-14,18-epoxyophiobola-7,19-dien-25-al Spiro[dicyclopenta[a,d]cyclooctene-3(2H),2'(3'H)-furan]-6-carboxaldehyde, 1,3a,4,4',5',6a,7,8,9,9a,10,10a-dodecahydro-9-hydroxy-3',9,10a-trimethyl-5'-(2-methyl-1-propen-1-yl)-7-oxo-, (3S,3'S,3aR,5E,5'R,6aS,9R,9aS,10aR)- (18R)-14,18-Epoxy-3-hydroxy-5-oxoophiobola-7,19-dien-25-al |
| Description | Ophiobolin A, a fungal metabolite and a phytotoxin, is a potent and irreversibly inhibitor of calmodulin-activated cyclic nucleotide phosphodiesterase, with an IC50 value of 9 μM. Ophiobolin A antimicrobial and anticancer activity[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 9 μM (calmodulin-activated cyclic nucleotide phosphodiesterase)[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 539.4±50.0 °C at 760 mmHg |
| Molecular Formula | C25H36O4 |
| Molecular Weight | 400.551 |
| Flash Point | 177.8±23.6 °C |
| Exact Mass | 400.261353 |
| PSA | 63.60000 |
| LogP | 3.75 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.554 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H312 + H332 |
| Precautionary Statements | Missing Phrase - N15.00950417-P261-P280 |
| Hazard Codes | Xn |
| Risk Phrases | 20/21/22 |
| Safety Phrases | S36 |
| RIDADR | UN 2811 6.1/PG 3 |
| RTECS | RL1576000 |