19309-14-9
| Name | (2E)-1-(2,4-Dihydroxy-6-methoxyphenyl)-3-phenyl-2-propen-1-one |
|---|---|
| Synonyms |
2-Propen-1-one, 1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenyl-, (2E)-
(E)-1-(2,4-Dihydroxy-6-methoxyphenyl)-3-phenyl-2-propen-1-one (2E)-1-(2,4-Dihydroxy-6-methoxyphenyl)-3-phenyl-2-propen-1-one (2E)-1-(2,4-Dihydroxy-6-methoxyphenyl)-3-phenylprop-2-en-1-one Cardamomin alpinetin chalcone Cardamonin |
| Description | Cardamonin is a novel antagonist of hTRPA1 cation channel with an IC50 of 454 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 454 nM (hTRPA1 cation channel)[1] |
| In Vitro | Cardamonin selectively blocksTRPA1 activation (IC50=454 nM) while does not interact with TRPV1 nor TRPV4 channel. Docking analysis of cardamonin demonstrates a compatible interaction with A-967079-binding site of TRPA1. Cardamonin does not significantly reduce HEK293 cell viability, nor does it impair cardiomyocyte constriction[1]. Cardamonin suppresses the expression of Tgase-2, cyclooxygenase-2 (COX-2), and p65 (nuclear factor-κB) in a concentration-dependent manner, and restores the expression of IκB in MG63 and Raw264.7 cells[2]. |
| In Vivo | Cardamonin (3-30 mg/kg, orally administered) significantly inhibits PBQ-induced writhing. CDN also produces a significant, dose-dependent increase in the withdrawal response latencies in carrageenan-induced hyperalgesia[2]. |
| Cell Assay | HEK293 cells are treated with cardamonin (0-90 μM). The cells treated in the absence of the test compound are the negative control. After incubated for 24 h, Cell Titer-Glo reagent is added to the cells and Luminescence is acquired on the plate reader[1]. |
| Animal Admin | Rats: The rats are divided into groups of six according to their nociceptive pressure thresholds, after which carrageenan (0.1 mL, 1%) is injected into the plantar surface of the left hind paw. The rats received vehicle or cardamonin (3-30 mg/kg) or indomethacin (3 mg/kg) orally 2 h after carrageenan injection and are evaluated for paw hyperalgesia 0, 1 and 2 h after administration of compounds. Indomethacin is used as a positive control[2]. Mice: Acute pain is induced by an intraperitoneal injection of 0.2 mL of 0.02% PBQ 54 min after oral administration of cardamonin. Six minutes after the PBQ injection, the total number of writhes is counted for 6 min. The control animals received an appropriate volume of dosing vehicle (80% saline, 10% ethanol and 10% Tween 80). Indomethacin is used as a positive control[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 484.5±45.0 °C at 760 mmHg |
| Molecular Formula | C16H14O4 |
| Molecular Weight | 270.280 |
| Flash Point | 182.7±22.2 °C |
| Exact Mass | 270.089203 |
| PSA | 66.76000 |
| LogP | 3.62 |
| Appearance | yellow |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.658 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: ≥20mg/mL |
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H400 |
| Precautionary Statements | P273 |
| Hazard Codes | N |
| Risk Phrases | 50/53 |
| Safety Phrases | 61-24/25 |
| RIDADR | UN 3077 9 / PGIII |
| HS Code | 2914509090 |
|
~10% 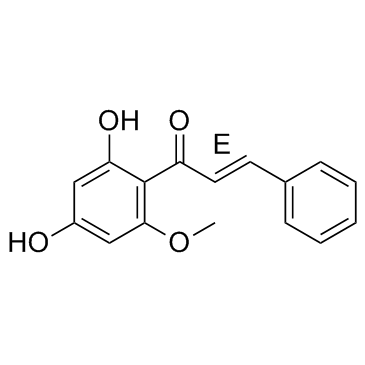
19309-14-9 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 20, # 1 p. 100 - 103 |
|
~% 
19309-14-9 |
| Literature: Chemische Berichte, , vol. 77/79, p. 99,105 Journal of Scientific and Industrial Research, , vol. 15B, p. 287,290 Chem.Abstr., , p. 3500 |
|
~% 
19309-14-9 |
| Literature: Journal of Scientific and Industrial Research, , vol. 15B, p. 287,290 Chem.Abstr., , p. 3500 |
|
~% 
19309-14-9 |
| Literature: Journal of Scientific and Industrial Research, , vol. 15B, p. 287,290 Chem.Abstr., , p. 3500 |
|
~% 
19309-14-9 |
| Literature: Journal of Scientific and Industrial Research, , vol. 15B, p. 287,290 Chem.Abstr., , p. 3500 |
| Precursor 5 | |
|---|---|
| DownStream 1 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |