1123231-07-1
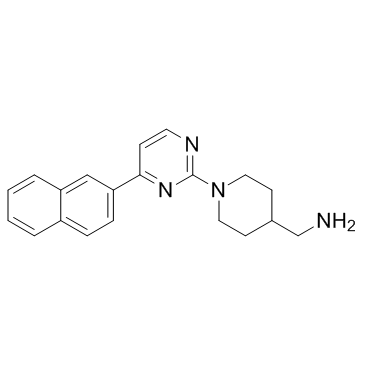
| Name | 1-{1-[4-(2-Naphthyl)-2-pyrimidinyl]-4-piperidinyl}methanamine |
|---|---|
| Synonyms |
1-{1-[4-(2-Naphthyl)-2-pyrimidinyl]-4-piperidinyl}methanamine
4-Piperidinemethanamine, 1-[4-(2-naphthalenyl)-2-pyrimidinyl]- 1-{1-[4-(2-Naphthyl)pyrimidin-2-yl]piperidin-4-yl}methanamine WAY-262611 |
| Description | WAY-262611 is a wingless β-Catenin agonist that increases bone formation rate with an EC50 of 0.63 μM in TCF-Luciferase assay. |
|---|---|
| Related Catalog | |
| Target |
EC50: 0.63 μM (β-Catenin)[1] |
| In Vitro | WAY-262611 has the most potent activity in the primary assay, low kinase inhibition potential, and high solubility[1]. |
| In Vivo | WAY-262611 has excellent pharmacokinetic properties and shows a dose dependent increase in the trabecular bone formation rate in ovariectomized rats following oral administration. Calvariae from wt mice treated with WAY-262611 shows statistically increased BFR, while similarly treated KO animals are no different from control. This indicates that WAY-262611 is acting via the Wnt β-catenin pathway and most likely through inhibition of Dkk-1[1]. |
| Animal Admin | Rats: WAY-262611 is dissolved in DMSO and diluted with saline for iv (Rats). WAY-262611 is prepared in 0.5% methylcellulose/2% Tween-80 for po OVX rats14 are treated orally with 5 (po, vehicle=0.5% methylcellulose/2% Tween-80, qd, 28 days) at four doses. Trabecular bone formation rate (BFR) in the tibia is established in all dose groups at the end of the in-life portion of the study. A clear dose response and activity as low as 0.3 mg/kg/day are observed[1]. Mice: To confirm activity via the Wnt pathway, the calvariae of wild type (wt) and Dkk-1 knockout (KO) mice are treated with 5 once a day for 7 days (DMSO solution, sc injection). The KO animals are not expected to respond because of the inherent inability to inhibit a missing target protein, while wild type animals with fully expressed Dkk-1 are expected to show a pharmacological response [1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 544.8±42.0 °C at 760 mmHg |
| Molecular Formula | C20H22N4 |
| Molecular Weight | 318.415 |
| Flash Point | 283.3±27.9 °C |
| Exact Mass | 318.184448 |
| PSA | 55.04000 |
| LogP | 4.09 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.638 |
| Storage condition | 2-8℃ |