111011-63-3
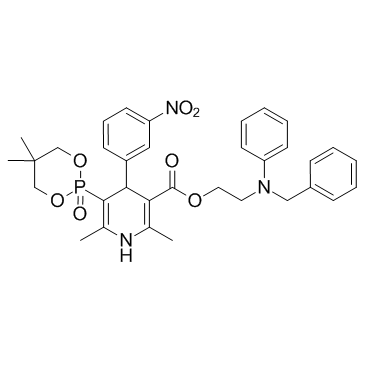
| Name | 2-(N-benzylanilino)ethyl 5-(5,5-dimethyl-2-oxo-1,3,2λ5-dioxaphosphinan-2-yl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3-carboxylate |
|---|---|
| Synonyms |
3-Pyridinecarboxylic acid, 5-(5,5-dimethyl-2-oxido-1,3,2-dioxaphosphorinan-2-yl)-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-, 2-[phenyl(phenylmethyl)amino]ethyl ester, (4S)-
2-[benzyl(phenyl)amino]ethyl 5-(5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinan-2-yl)-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3-pyridinecarboxylate Efonidipine HCl 3-(benzyl-(phenyl)amino)ethyl-5-(5,5-dimethyl-2-oxo-1,3-dioxaphosphorinane-2-yl)-1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-pyridine 2-[Benzyl(phenyl)amino]ethyl (4S)-5-(5,5-dimethyl-2-oxido-1,3,2-dioxaphosphinan-2-yl)-2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydro-3-pyridinecarboxylate Efonidipine [INN] Efonidipine |
| Description | Efonidipine(NZ-105) is a dual T-type and L-type calcium channel blocker (CCB).IC50 value: Target: calcium channel blockerin vitro: Efonidipine and nifedipine, but not other examined CCBs, also increased the N(6), 2'-O-dibutyryladenosine 3',5'-cyclic monophosphate (dbcAMP)-induced StAR mRNA, which reflects the action of adrenocorticotropic hormone, and efonidipine and R(-)-efonidipine enhanced the dbcAMP-induced DHEA-S production in NCI-H295R adrenocortical carcinoma cells [1]. I(Ca(T)) was blocked mainly by a tonic manner by nifedipine, by a use-dependent manner by mibefradil, and by a combination of both manners by efonidipine. IC50s of these Ca2+ channel antagonists to I(Ca(T)) and L-type Ca2+ channel current (I(Ca(L))) were 1.2 micromol/l and 0.14 nmol/l for nifedipine; 0.87 and 1.4 micromol/l for mibefradil, and 0.35 micromol/l and 1.8 nmol/l for efonidipine, respectively [4].in vivo: Twenty hypertensive patients on chronic hemodialysis were given efonidipine 20-60 mg twice daily and amlodipine 2.5-7.5 mg once daily for 12 weeks each in a random crossover manner. The average blood pressure was comparable between the efonidipine and amlodipine periods (151 + or - 15/77 + or - 8 versus 153 + or - 15/76 + or - 8 mmHg). The pulse rate did not change significantly during the administration periods [2]. In the UM-X7.1 group, EFO treatment significantly attenuated the decrease of LVEF without affecting blood pressure compared with the vehicle group. EFO treatment decreased heart rate (by approximately 10%) in both groups [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 746.9±60.0 °C at 760 mmHg |
| Molecular Formula | C34H38N3O7P |
| Molecular Weight | 631.655 |
| Flash Point | 405.5±32.9 °C |
| Exact Mass | 631.244751 |
| PSA | 132.73000 |
| LogP | 6.99 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.625 |
| Storage condition | -20℃ |
|
~% 
111011-63-3 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2362 - 2369 |
|
~% 
111011-63-3 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2362 - 2369 |
|
~% 
111011-63-3 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 9 p. 2362 - 2369 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |