146362-70-1
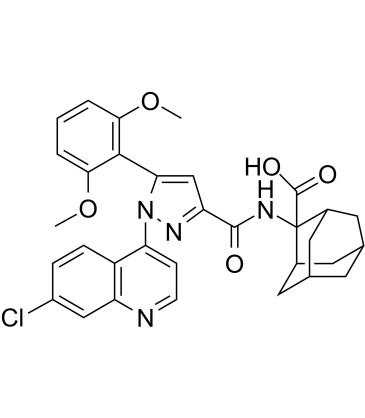
| Name | 2-[[1-(7-chloroquinolin-4-yl)-5-(2,6-dimethoxyphenyl)pyrazole-3-carbonyl]amino]adamantane-2-carboxylic acid |
|---|---|
| Synonyms |
Reminertant
merclinertant SR48692 2-({[1-(7-Chloroquinolin-4-yl)-5-(2,6-dimethoxyphenyl)-1H-pyrazol-3-yl]carbonyl}amino)adamantane-2-carboxylic acid UNII-5JBP4SI96H 2-({[1-(7-Chloro-4-quinolinyl)-5-(2,6-dimethoxyphenyl)-1H-pyrazol-3-yl]carbonyl}amino)-2-adamantanecarboxylic acid Meclinertant Tricyclo[3.3.1.1]decane-2-carboxylic acid, 2-[[[1-(7-chloro-4-quinolinyl)-5-(2,6-dimethoxyphenyl)-1H-pyrazol-3-yl]carbonyl]amino]- |
| Description | Meclinertant (SR 48692) is a potent, selective, nonpeptide and orally active neurotensin receptor 1 (NTS1) antagonist. In human colon carcinoma (HT-29) cells, Meclinertant competitively antagonizes neurotensin-induced intracellular Ca2+ mobilization with a pA2 values of 8.13. Meclinertant has anxiolytic, anti-addictive and memory-impairing effects[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Neurotensin receptor 1 (NTS1)[1] |
| In Vitro | In vitro, Meclinertant (SR 48692) competitively inhibits 125I-labeled neurotensin binding to the high-affinity binding site present in brain tissue from various species with IC50 values of 0.99 nM (guinea pig), 4.0 nM (rat mesencephalic cells), 7.6 nM (COS-7 cells transfected with the cloned high-affinity rat brain receptor), 13.7 nM (newborn mouse brain), 17.8 nM (newborn human brain), 8.7 nM (adult human brain), and 30.3 nM (HT-29 cells). Meclinertant also displaces 125I-labeled neurotensin from the low-affinity levocabastine-sensitive binding sites but at higher concentrations (34.8 nM for adult mouse brain and 82.0 nM for adult rat brain)[1]. In guinea pig striatal slices, Meclinertant blocks K+-evoked release of [3H]dopamine stimulated by neurotensin with a potency (IC50 = 0.46 nM) that correlates with its binding affinity[1]. |
| In Vivo | Meclinertant (SR 48692) treatment reverses at 80 μg/kg the turning behavior induced by intrastriatal injection of neurotensin in mice and with a long duration of action (6 hours)[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 781.2±60.0 °C at 760 mmHg |
| Molecular Formula | C32H31ClN4O5 |
| Molecular Weight | 587.065 |
| Flash Point | 426.2±32.9 °C |
| Exact Mass | 586.198303 |
| PSA | 119.06000 |
| LogP | 4.55 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.729 |
| Storage condition | -20°C |
| RTECS | YD1988500 |
|---|
| Precursor 5 | |
|---|---|
| DownStream 0 | |