100-33-4
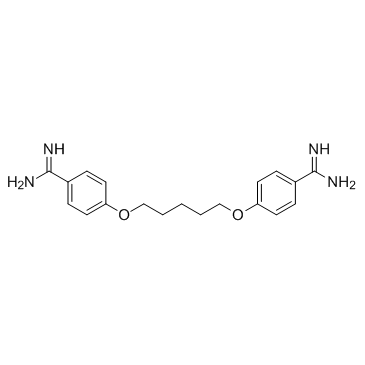
| Name | pentamidine |
|---|---|
| Synonyms |
4,4'-[Pentane-1,5-diylbis(oxy)]dibenzenecarboximidamide
4,4'-(Pentane-1,5-diylbis(oxy))dibenzimidamide pentamidine EINECS 202-841-0 4,4'-[1,5-Pentanediylbis(oxy)]dibenzenecarboximidamide |
| Description | Pentamidine(MP-601205) is an antimicrobial agent.Target: AntiparasiticPentamidine has a potent in vitro antiprotozoal activity. Pentamidine displays cytotoxic activity against L. infantum promastigotes with IC50 of 2.5 μM. 2.5 μM Pentamidine induces early programmed cell death in 49.6% of L. infantum promastigotes. 2.5 μM Pentamidine induces a notorious decrease in promastigotes in both G1 and S phases relative to the control-untreated samples (G1:77.0 vs 15.0%; S:11.0 vs 2.4% for control- and pentamidine-treated promastigotes, resp). Pentamidine is able to bind with calf-thymus DNA (CT-DNA) and induces conformational changes in the DNA double helix. Pentamidine also binds with ubiquitin to modifiy the β-cluster of ubiquitin [1]. Pentamidine is an inhibitor of phosphatase of regenerating liver (PRLs). 1 μg/mL of Pentamidine complete inhibits the activity of recombinant PTP1B in dephosphorylating a phos-photyrosine peptide. 10 μg/mL of Pentamidine completely inhibits the activities of recombinant PRL-1, PRL-2 and PRL-3 in dephosphorylating a phosphotyrosine peptide substrate. Incubation with Pentamidine (1 μg/mL) for 48 h reduces the activity of intracellular PRL phosphatases in transfected NIH3T3 cells by more than 85%. 10 μg/mL Pentamidine completely inhibits the growth of melanoma cell line (WM9), prostate carcinoma cell line (DU145 and C4-2), ovarian carcinoma cell line (Hey), colon carcinoma cell line (WM480), and lung carcinoma cell line (A549) which all express endogenous PRLs [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 539.4±60.0 °C at 760 mmHg |
| Melting Point | 186ºC (dec.) |
| Molecular Formula | C19H24N4O2 |
| Molecular Weight | 340.419 |
| Flash Point | 280.0±32.9 °C |
| Exact Mass | 340.189911 |
| PSA | 118.20000 |
| LogP | 2.47 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.593 |
| Storage condition | -20°C Freezer, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| RIDADR | UN 3249 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2925290090 |
|
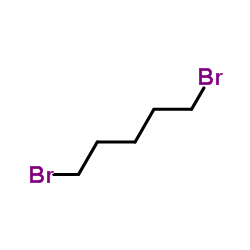
~% 
100-33-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1252 - 1257 |
|
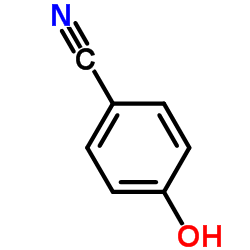
~% 
100-33-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1252 - 1257 |
|
~% 
100-33-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 4 p. 1252 - 1257 |
| HS Code | 2925290090 |
|---|---|
| Summary | 2925290090 other imines and their derivatives; salts thereof。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |