17086-76-9
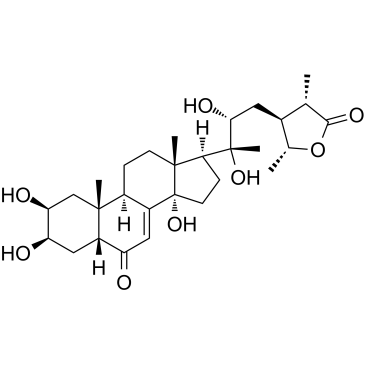
| Name | cyasterone |
|---|---|
| Synonyms |
(2β,3β,5β,22R,24S,25S,28R)-2,3,14,20,22-Pentahydroxy-26,28-epoxystigmast-7-ene-6,26-dione
(2β,3β,5β,23R,24ξ,25S,28R)-2,3,14,20,23-Pentahydroxy-26,28-epoxystigmast-7-ene-6,26-dione Stigmast-7-ene-6,26-dione, 26,28-epoxy-2,3,14,20,23-pentahydroxy-, (2β,3β,5β,23R,24ξ,25S,28R)- cyasteron Stigmast-7-ene-6,26-dione, 26,28-epoxy-2,3,14,20,22-pentahydroxy-, (2β,3β,5β,22R,24S,25S,28R)- Cyasterone |
| Description | Cyasterone, a natural EGFR inhibitor, mainly isolated from Ajuga decumbens Thunb (Labiatae).Cyasterone manifests anti-proliferation effect by induced apoptosis and cell cycle arrests. Cyasterone may serves as a clinical therapeutic anti-tumor agent against human tumors[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Cyasterone (0-100μM; 24hours) inhibits cell growth in a concentration-and time-dependent manner, IC50 values are 38.50 mg/mL, 32.96 mg/mL in A549 and MGC823 cells ,respectively[1]. Cell Viability Assay[1] Cell Line: A549, HCT116, MGC823 cells Concentration: 0-100 μM Incubation Time: 24 hours Result: Inhibited the growth of 3 human cancer lines A549, HCT116, MGC823 cells. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 742.8±60.0 °C at 760 mmHg |
| Molecular Formula | C29H44O8 |
| Molecular Weight | 520.655 |
| Flash Point | 242.0±26.4 °C |
| Exact Mass | 520.303589 |
| PSA | 144.52000 |
| LogP | -0.25 |
| Vapour Pressure | 0.0±5.6 mmHg at 25°C |
| Index of Refraction | 1.600 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Safety Phrases | 24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | WJ2510000 |