491-80-5
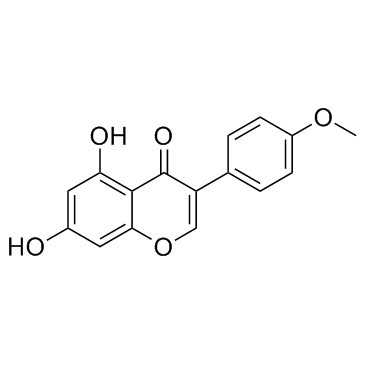
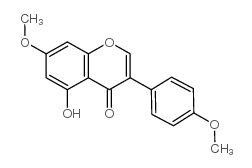
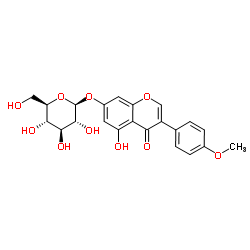
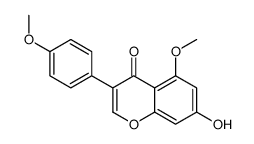
| Name | biochanin A |
|---|---|
| Synonyms |
Biochanin A
Genistein 4'-Methyl Ether 5,7-Dihydroxy-4'-methoxyisoflavone Genistein 4′-methyl ether 5,7-Dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one MFCD00006839 UNII-U13J6U390T 5,7-Dihydrox -4'-methoxyisoflavone 5,7-dihydroxy-4'-methoxy-Isoflavone (8CI) 5,7-Dihydroxy-4′-methoxyisoflavone 5,7-Dihydrox-4'-methoxyisoflavone olmelin 5,7-dihydroxy-4'-methoxy-Isoflavone EINECS 207-744-7 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-methoxyphenyl)- 5,7-dihydroxy-3-(4-methoxyphenyl)-4-chromenone |
| Description | Biochanin A is a naturally occurring fatty acid amide hydrolase (FAAH) inhibitor, which inhibits FAAH with IC50s of 1.8, 1.4 and 2.4 μM for mouse, rat, and human FAAH, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 1.8 μM (mouse FAAH), 1.4 μM (rat FAAH), 2.4 μM (human FAAH)[1] |
| In Vitro | Biochanin A inhibits the hydrolysis of 0.5 µM AEA by mouse, rat and human FAAH with IC50 s of 1.8, 1.4 and 2.4 µM respectively. FAAH is inhibited by Biochanin A with a pIC50 value of 6.21±0.02, corresponding to an IC50 value of 0.62 µM. Biochanin A produces significant inhibition of the URB597-sensitive tritium retention at high nanomolar-low micromolar concentrations. Experiments are run with human FAAH and 0.5 µM [3H]AEA with assay conditions giving these higher utilization rates, the activity is still inhibited by Biochanin A, Genistein, Formononetin and Daidzein in the low micromolar range (IC50s of 6.0, 8.4, 12 and 30 µM, respectively)[1]. |
| In Vivo | Biochanin A is tested at doses of 30, 100 and 300 µg. The highest dose also reduced formalin-induced ERK phosphorylation in a manner antagonized by AM251. Thus, Biochanin A behaved like URB597 after local administration to the paw. In anaesthetized mice, URB597 (30 µg i.pl.) and Biochanin A (100 µg i.pl.) both inhibit the spinal phosphorylation of extracellular signal-regulated kinase produced by the intraplantar injection of formalin. The effects of both compounds are significantly reduced by the CB1 receptor antagonist/inverse agonist AM251 (30 µg i.pl.). Biochanin A (15 mg/k i.v.) does not increase brain AEA concentrations, but produces a modest potentiation of the effects of 10 mg/kg i.v. AEA in the tetrad test. Biochanin A (15 mg/kg i.v.) is without effects on its own, but significantly potentiates the effects of AEA (10 mg/kg i.v.)[1]. |
| Kinase Assay | For experiments with FAAH, rat liver homogenates, mouse brain homogenates and membranes from COS7 cells transfected with the human enzyme are used. Frozen (−80°C) livers from adult C57BL/6 mice and frozen brains (minus cerebella) from adult Wistar or Sprague-Dawley rats are thawed and homogenized in 20 mM HEPES, 1 mM MgCl2, pH 7. The homogenates are centrifuged at ~35000×g for 20 min at 4°C. After resuspension in buffer followed by recentrifugation and a second resuspension in buffer, the pellets are incubated at 37°C for 15 min. This incubation is undertaken in order to hydrolyse all endogenous FAAH substrates. The homogenates are then centrifuged as above, recentrifuged and resuspended in 50 mM Tris-HCl buffer, pH 7.4, containing 1 mM EDTA and 3 mM MgCl2. The homogenates are then frozen at −80°C in aliquots until used for assay. FAAH is assayed in the homogenates and in the COS7 cell membranes using 0.5 µM (unless otherwise stated) [3H]AEA labelled in the ethanolamine part of the molecule. Blank values are obtained by the use of buffer rather than homogenate. In the experiments comparing effects of Biochanin A upon FAAH and FAAH-2, the same assay is used but with 16 nM [3H]oleoylethanolamide ([3H]OEA) as substrate and with an incubation phase at room temperature. The choice of OEA rather than AEA for FAAH-2 is motivated by the relative rates of hydrolysis: OEA is metabolized four times faster than AEA by FAAH-2, whereas for FAAH the rate of hydrolysis of OEA is about a third of that for AEA. When 0.5 µM [3H]AEA is used as substrate, assay conditions for rat brain and mouse liver are chosen so that <10% of added substrate is metabolized. For the human FAAH samples, <5% of the [3H]AEA is metabolized in all cases. For 16 nM [3H]OEA, a limited supply of an expensive ligand meant that optimization is not possible, and the amount of substrate utilized is higher (34±1 and 0.5±0.1% for FAAH and its corresponding mock-transfected, respectively; 40±2 and 21±0.4 for FAAH-2 and its corresponding mock-transfected respectively)[1]. |
| Animal Admin | Mice[1] ICR mice are used for the behavioural tests measuring spontaneous activity (over a 10 min testing period), rectal temperature, ring immobility (over a 5 min testing period) and nociceptive threshold (tail flick tests). AEA and Biochanin A are dissolved in a vehicle consisting of ethanol, Emulphor-620 and physiological saline in a ratio of 1:1:18 v/v, and administered i.v. to the animals via the tail vein (injection volume 10 µL/g body weight). The degree of antinociception is expressed as percentage of maximum possible effect (%MPE), defined as [(test-control time)/(10-control time)]×100. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 518.6±50.0 °C at 760 mmHg |
| Melting Point | 210-213 °C(lit.) |
| Molecular Formula | C16H12O5 |
| Molecular Weight | 284.263 |
| Flash Point | 198.3±23.6 °C |
| Exact Mass | 284.068481 |
| PSA | 79.90000 |
| LogP | 3.14 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.669 |
| Storage condition | 0-6°C |
| Water Solubility | acetone: 10 mg/mL, clear, brown |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2932999099 |
| Precursor 10 | |
|---|---|
| DownStream 7 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |







![[5-acetyloxy-3-(4-methoxyphenyl)-4-oxochromen-7-yl] acetate structure](https://image.chemsrc.com/caspic/415/54443-59-3.png)