11021-13-9
| Name | ginsenoside Rb2 |
|---|---|
| Synonyms |
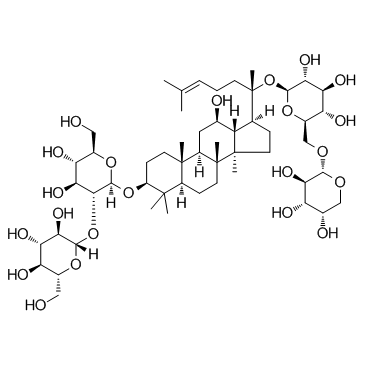
20-((6-O-α-L-Arabinopyranosyl-β-D-glucopyranosyl)oxy)-12β-hydroxydammar-24-en-3β-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside
GinsenosideRb2 (2S,3R,4S,5S,6R)-2-{[(2R,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxyméthyl)-2-({(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-4,4,8,10,14-pentaméthyl-17-[(2S)-6-méthyl-2-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2S,3R,4S,5S)-3,4,5-trihydroxytétrahydro-2H-pyran-2-yl]oxy}méthyl)tétrahydro-2H-pyran-2-yl]oxy}-5-heptèn-2-yl]hexadécahydro-1H-cyclopenta[a]phénanthrén-3-yl}oxy)tétrahydro-2H-pyran-3-yl]oxy}-6-(hydroxyméthyl)tétrahydro-2H-pyran-3,4,5-triol (3β,12β)-20-{[6-O-(α-L-Arabinopyranosyl)-β-D-glucopyranosyl]oxy}-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside β-D-Glucopyranoside, (3β,12β)-20-[(6-O-α-L-arabinopyranosyl-β-D-glucopyranosyl)oxy]-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl- GINSENOSIDE RB2 (2S,3R,4S,5S,6R)-2-{[(2R,3R,4S,5S,6R)-4,5-Dihydroxy-6-(hydroxymethyl)-2-({(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-4,4,8,10,14-pentamethyl-17-[(2S)-6-methyl-2-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2S,3R,4S,5S)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl]oxy}methyl)tetrahydro-2H-pyran-2-yl]oxy}-5-hepten-2-yl]hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl}oxy)tetrahydro-2H-pyran-3-yl]oxy}-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (3β,12β)-20-{[6-O-(α-L-Arabinopyranosyl)-β-D-glucopyranosyl]oxy}-12-hydroxydammar-24-en-3-yl-2-O-β-D-glucopyranosyl-β-D-glucopyranoside ginsenosidec MFCD00221755 EINECS 234-251-4 |
| Description | Ginsenoside Rb2 is one of the main bioactive components of ginseng extracts. Rb2 can upregulate GPR120 gene expression. |
|---|---|
| Related Catalog | |
| Target |
GPR120[1] |
| In Vitro | Ginsenoside Rb2 pre-treatment enhances the anti-inflammatory effect of α-linolenic acid (ALA) and that the enhancing effect is strictly dependent on GPR120 activation. Ginsenoside Rb2 exerts anti-inflammatory effect in lipopolysaccharide (LPS)-stimulated mouse macrophage RAW264.7 cells in vitro by increasing GPR120 expression and subsequently enhancing ω-3 fatty acid-induced GPR120 activation. Ginsenoside Rb2 improves glucose metabolism in hepatocytes by activating AMPK and reduces cholesterol and triacylglycerol levels in 3T3-L1 cells by reducing oxidative damage. Ginsenoside Rb2 exerts anti-apoptosis effects in murine bone marrow-derived mesenchymal stem cells (BMMSCs). MTT assay results show no obvious cytotoxicity of Ginsenoside Rb2 (up to 100 μM) toward RAW264.7 cells in the absence or presence of ALA. The influence of Rb2 on GPR120 expression in RAW264.7 macrophages is investigated by treating the cells with Ginsenoside Rb2 (0.1-100 μM) for 12 h followed by harvesting and lysis. Subsequent Western blot analysis shows that expression of GPR120 is dose-dependently upregulated by Ginsenoside Rb2. Real-time PCR results indicate that incubation of RAW264.7 macrophages with Ginsenoside Rb2 (10 μM) for 12 h leads to a 2.8-fold increase in GPR120 mRNA expression. In addition, this increase in GPR120 expression stimulated by Ginsenoside Rb2 is time dependent and begins as early as 6 h. These results indicate that Rb2 upregulates GPR120 expression in a dose- and time-dependent manner in RAW264.7 macrophages[1]. |
| In Vivo | Ginsenoside Rb2 is an antiviral reagent to protect against rotavirus (RV) infection. When various dosages of Ginsenoside Rb2 (25 to 250 mg/kg) are administered 3, 2 or 1 days before virus challenge, treatment with this Ginsenoside at the dosage of 75 mg/kg 3 days before virus infection most effectively reduces rotavirus (RV) -induced diarrhea. In addition, consecutive administration of Ginsenoside Rb2 (75 mg/kg) 3, 2, and 1 day before virus infection is more effective than single administration on day-3. The consecutive administration of Ginsenoside Rb2 also reduces virus titers in the bowels of RV-infected mice[2]. |
| Cell Assay | Cell viability is determined using the MTT assay. RAW264.7 cells (2×104 cells/well) are plated in 96-well plates and cultured overnight in growth medium. The cells are then incubated with Ginsenoside Rb2 (0.1, 1, 10 and 100 μM) at the indicated concentrations in the absence or presence of ALA for 48 h before addition of the MTT reagent (0.5 mg/mL). After incubation for 4 h, the medium is removed, and the formazan crystals formed are dissolved with 100 μL DMSO. Absorbance at a wavelength of 492 nm is measured using a FlexStation 3[1]. |
| Animal Admin | Mice[2] Groups of five BALB/c newborn mice are inoculated p.o. with RV-SA11 (1.5×106 plaque-forming units (PFU)/mouse) and administered p.o. with the indicated doses of Ginsenoside Rb2 on the indicated days. In order to examine the protective effect of Ginsenoside Rb2 on RV infection, newborn mice are treated orally with 75 mg/kg of this Ginsenoside 3, 2 or 1 day before RV infection, and the severity of diarrhea of RV-infected mice i calculated. In addition, in an experiment in which various doses of Ginsenoside Rb2 ranging from 25 to 250 mg/kg are administered to mice 3 days before RV infection, Ginsenoside Rb2 at the dose of 75 mg/kg elicits higher protective activity compared to either of the dose of 25 to 250 mg/kg. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 1117.1±65.0 °C at 760 mmHg |
| Melting Point | 197-199ºC |
| Molecular Formula | C53H90O22 |
| Molecular Weight | 1079.269 |
| Flash Point | 629.4±34.3 °C |
| Exact Mass | 1078.592407 |
| PSA | 357.06000 |
| LogP | 4.73 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.622 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
11021-13-9 |
| Literature: Chemistry and Biodiversity, , vol. 8, # 10 p. 1853 - 1863 |

