509-15-9
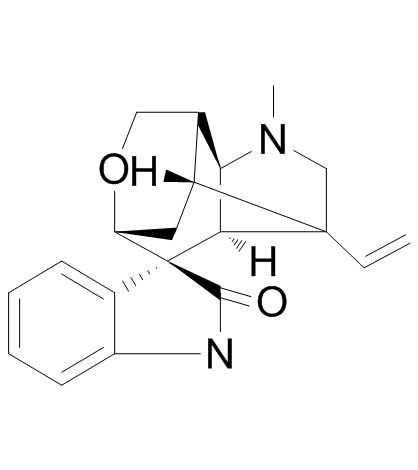
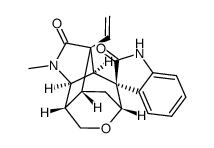
| Name | Gelsemine |
|---|---|
| Synonyms |
GELSAMINE
8abeta,9s*,10s*))-8alph GESEMINE (2'S,3S,5'S,6'R,8'R,11'S)-4'-Methyl-2'-vinylspiro[indole-3,7'-[9]oxa[4]azatetracyclo[6.3.1.0.0]dodecan]-2(1H)-one gelsemine free base Gelsemin EINECS 208-095-2 |
| Description | Gelsemine, an alkaloid from the Chinese herb Gelsemium elegans, is effective in mitigating chronic pain. Antinociceptive and hypnotic effects. |
|---|---|
| Related Catalog | |
| In Vivo | Gelsemine is an effective agent for treatment of both neuropathic pain and sleep disturbance in partial sciatic nerve ligation (PSNL) mice. Gelsemine (4 mg/kg) exerts analgesic effects on PSNL-induced mechanical allodynia and thermal hyperalgesia. In PSNL mice, Gelsemine (2 and 4 mg/kg) increases the mechanical threshold for 4 h and prolonged the thermal latencies for 3 h. Furthermore, Gelsemine (4 mg/kg, administered at 6:30 AM) increases non-rapid eye movement (non-REM, NREM) sleep, decreases wakefulness, but does not affect rapid eye movement (REM) sleep during the first 3 h in PSNL mice[1]. Immunohistochemical study shows that PSNL increases c-Fos expression in the neurons of the anterior cingulate cortex, and Gelsemine (4 mg/kg) decreases c-Fos expression by 58%[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 493.4±45.0 °C at 760 mmHg |
| Melting Point | 181-183ºC |
| Molecular Formula | C20H22N2O2 |
| Molecular Weight | 322.401 |
| Flash Point | 252.2±28.7 °C |
| Exact Mass | 322.168121 |
| PSA | 41.57000 |
| LogP | 3.08 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.673 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | T+ |
|---|---|
| Risk Phrases | 23/24/25-26/27/28 |
| Safety Phrases | 36/37/39-45-28 |
| RIDADR | UN 1544 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | LX9100000 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
| Precursor 1 | |
|---|---|
| DownStream 0 | |