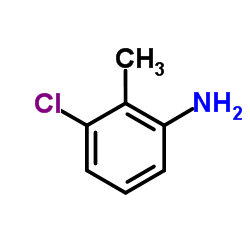
13710-19-5
| Name | tolfenamic acid |
|---|---|
| Synonyms |
Clotam
N-(3-Chloro-ortho-tolyl) anthranilic acid 2-[(3-Chloro-2-methylphenyl)amino]benzoic acid Tolfenamic acid MFCD00133865 N-(3-Chloro-o-tolyl)-anthranilic acid EINECS 237-264-3 Tolfedine 2-((3-Chloro-2-methylphenyl)amino)benzoic acid 2-(3-Chloro-o-toluidino)benzoic Acid N-(3-Chloro-ortho-tolyl)anthranilic acid N-(2-Methyl-3-chlorophenyl)anthranilic acid Benzoic acid, 2-[(3-chloro-2-methylphenyl)amino]- N-(3-Chloro-2-methylphenyl)anthranilic Acid 2 (3-Chloro-2-methylanilino)benzoic acid 2-(3-chloro-2-methylanilino)benzoic acid Anthranilic acid, N-(3-chloro-o-tolyl)- N-(3-chloro-o-tolyl)anthranilic acid Tolfine |
| Description | Tolfenamic Acid is a non-steroidal anti-inflammatory and anti-cancer agent, selectively inhibits COX-2, with an IC50 of 13.49 μM (3.53 μg/mL) in LPS-treated (COX-2) canine DH82 monocyte/macrophage cells, but shows no effect on COX-1. |
|---|---|
| Related Catalog | |
| Target |
COX-2:13.49 μM (IC50, in cell) |
| In Vitro | Tolfenamic Acid is a nonsteroidal antiinflammatory agent, selectively inhibits COX-2, with an IC50 of 13.49 μM (3.53 μg/mL) in LPS-treated (COX-2) canine DH82 monocyte/macrophage cells, but shows no effect on COX-1[1]. Tolfenamic Acid (100 μM) inhibits >70% of cell viability of BE3, OE33, and SKGT5. Tolfenamic Acid also acts as a potent Sp protein inhibitor, decreases Sp1 and Sp4 and suppresses c-Met expression in esophageal cancer cells BE3 and SKGT5[2]. Tolfenamic Acid (50 μM) significantly affects gene expression in L3.6pl cells, and downregulates CENPF, KIF20A, LMNB1, MYB, SKP2, CCNE2, and DDIT3[3]. |
| In Vivo | Tolfenamic Acid (50 mg/kg 3 times/wk, p.o.) inhibits tumor formation and tumor incidence in N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumor model. Tolfenamic Acid also causes decreases in tumor multiplicity and tumor volume in rats treated with NMBA[2]. |
| Cell Assay | All cells are grown in media (RPMI1640) supplemented with 5% serum and cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2. Twenty four hours after seeding, cells are treated with vehicle (0.1% DMSO) or various concentrations of Tolfenamic Acid (25/50/100 μM). Cell viability assays are conducted at 24, 48 and 72 h post-treatment. Cells are treated with 50 μM Tolfenamic Acid and the cell pellets are harvested at 48 h post-treatment. These pellets are used to prepare cell lysates that are used in Western blot analyses[2]. |
| Animal Admin | Mice[2] The Fischer 344 (F-344) rat model of esophageal SCC are initially housed two per cage, but eventually separated to one per cage due to increase in size, and are maintained under standard, humane conditions (20±2°C, 50±10% relative humidity, 12-h light/dark cycles). Food and water are provided ad libitum. Body weights are recorded at the time of each dosing. Two weeks after arrival in the animal facility, the rats are randomLy assigned into 4 groups: C: NMBA (1-5 week); NTA: (NMBA 1-5 week and then Tolfenamic Acid 6-25 week); NC: Negative control (vehicle); and TA: Tolfenamic Acid negative control. Control (C) and NTA groups are injected s.c. with NMBA (0.5 mg/kg) in 0.2 mL vehicle three times per week for 5 weeks while negative control groups are injected with vehicle alone. NTA and Tolfenamic Acid groups also receive 50 mg/kg Tolfenamic Acid by oral gavage from week 6 through week 25. After the 25th week, the animals are sacrificed, esophagi are cut open longitudinally, and surface tumors are mapped and counted. The number and the size of lesions, including polyps are recorded and images captured. Tumor volume is calculated using the formula for a prolate spheroid: length × width × height × p/6[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 405.4±40.0 °C at 760 mmHg |
| Melting Point | 210-214°C |
| Molecular Formula | C14H12ClNO2 |
| Molecular Weight | 261.704 |
| Flash Point | 199.0±27.3 °C |
| Exact Mass | 261.055664 |
| PSA | 49.33000 |
| LogP | 5.76 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.658 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | CB2687500 |
| HS Code | 2922499990 |
|
~%
Detail
|
| Literature: US4092430 A1, ; |
|
~39% 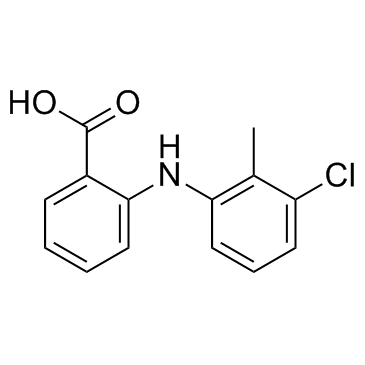
13710-19-5 |
| Literature: Kovala-Demertzi, Dimitra; Hadjipavlou-Litin, Dimitra; Primikiri, Alexandra; Staninska, Malgorzata; Kotoglou, Chronis; Demertzis, Mavroudis A. Chemistry and Biodiversity, 2009 , vol. 6, # 6 p. 948 - 960 |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |