| Description |
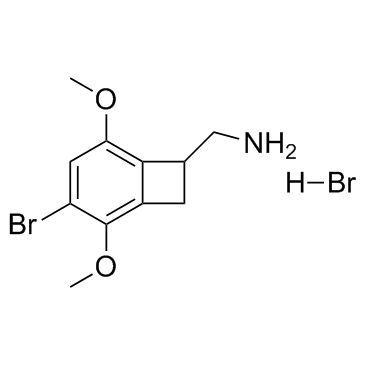
TCB2 is an agonist of serotonin 5-HT2A receptor.
|
| Related Catalog |
|
| Target |
5-HT2A Receptor
|
| In Vitro |
The selective 5-HT2A agonist TCB2 at 30 nM produces a marked shift to the right in the competition curve obtained with the 5-HT1A receptor agonist ipsapirone from [3H]-8-OHDPAT binding sites in both areas of the brain. A significant increase in the mean Ki value is obtained with TCB2, demonstrating a reduction in the affinity of the high-affinity 5-HT1A agonist binding sites by the 5-HT2A agonist in both regions. In the case of hippocampus, there are also indications for antagonistic effects of ketanserin[2].
|
| In Vivo |
In an initial dose-response study, TCB2 (0.5, 1.0, and 2.5 mg/kg) and DOI (0.5, 1.0, 2.5, and 5.0 mg/kg) induce head twitches in C57BL/6J mice in a dose-dependent manner compared to vehicle. Head twitches induced by TCB2 and DOI are similar across the doses, with the exception that at the highest dose (5.0 mg/kg), TCB2 induces half as many head twitches as does DOI (p=0.021). Confirming 5-HT2A mediation of the head twitch response, in two separate studies, pretreatment with the selective 5-HT2A antagonist MDL 11,939 (1.0 mg/kg) block head twitches induced by DOI (2.5 mg/kg) and by TCB2 (2.5 mg/kg; p<0.0001). Compared to vehicle, corticosterone levels are increased following TCB2 (5.0 mg/kg, p=0.01; 10.0, p=0.012; with a trend following 2.5 mg/kg, p=0.079) and DOI (10.0 mg/kg, p=0.035)[1].
|
| Animal Admin |
Mice[1] Male C57BL/6J mice and SERT+/+ (+/−, −/−) mice (25-30 g) are used. TCB2 is dissolved in distilled water, MDL 11,939 is dissolved in a few drops of acetic acid and prepared in distilled water, and DOI is dissolved in saline. All drugs and their vehicles are administered via intraperitoneal injection at a volume of 10 ml/kg. Following 15 min of habituation in a plexiglass container, mice are administered the test drug. Head twitches were recorded for five 1-min periods over a 30-min period; the scores from the five 1-min periods are summed together. In two separate experiments, mice are pretreated with the selective 5-HT2A antagonist MDL 11,939 (1.0 mg/kg) or its vehicle 30 min prior to administration of DOI or TCB2 (2.5 mg/kg). Finally, head twitches are assessed in SERT+/+ (+/−, −/−) mice administered TCB2. C57BL/6J mice are injected with vehicle, TCB2 (1.0 or 2.5 mg/kg) or DOI (1.0 or 2.5 mg/kg). Following administration of vehicle, TCB2 (1.0, 2.5, or 5.0 mg/kg) or DOI (1.0, 2.5, or 5.0 mg/kg), mice are placed in the test cage. In a final feeding study, mice are pretreated with vehicle or MDL 11,939 (1.0 mg/kg) followed 30 min later by an intermediate dose of TCB2 (3.75 mg/kg)[1].
|
| References |
[1]. Fox MA, et al. The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl). 2010 Sep;212(1):13-23. [2]. Dasiel O. Borroto-Escuela, et al. Existence of Brain 5-HT1A–5-HT2A Isoreceptor Complexes with Antagonistic Allosteric Receptor–Receptor Interactions Regulating 5-HT1A Receptor Recognition. ACS Omega. 2017 Aug 31; 2(8): 4779–4789.
|