135159-51-2
| Name | Sarpogrelate Hydrochloride |
|---|---|
| Synonyms |
Butanedioic acid, mono(2-(dimethylamino)-1-((2-(2-(3-methoxyphenyl)ethyl)phenoxy)methyl)ethyl) ester, hydrochloride
4-[1-(dimethylamino)-3-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]propan-2-yl]oxy-4-oxobutanoic acid,hydrochloride Butanedioic acid, mono[2-(dimethylamino)-1-[[2-[2-(3-methoxyphenyl)ethyl]phenoxy]methyl]ethyl] ester, hydrochloride (1:1) 4-{[1-(Dimethylamino)-3-{2-[2-(3-methoxyphenyl)ethyl]phenoxy}propan-2-yl]oxy}-4-oxobutanoic acid hydrochloride (1:1) 4-{[1-(Dimethylamino)-3-{2-[2-(3-methoxyphenyl)ethyl]phenoxy}-2-propanyl]oxy}-4-oxobutanoic acid hydrochloride (1:1) UNII:FQN8N8QP1B Sarpogrelate HCl Sarpogrelate (hydrochloride) |
| Description | Sarpogrelate(MCI-9042) hydrochloride, a selective 5-HT2 antagonist, has been widely used as an anti-platelet agent for the treatment of PAD.Target: 5-HT2 RecepterSarpogrelate is a drug which acts as an antagonist at the 5HT2A and 5-HT2B receptors. Sarpogrelate was shown to have the same affinity as ritanserin for 5-HT2A receptors, with a Ki value of 8.39 nM [1]. Sarpogrelate lacked prominent 5-HT1-like, 5-HT3, beta, H1, H2 and M3 antagonist activity and weakly blocked alpha 1-adrenoceptors (pKB = 6.30). (S)-M-1 showed weak affinity for 5-HT1-like receptors (pKB = 6.30), alpha 1- (pKB = 6.80) and beta- (pKB = 6.54) adrenoceptors, while (R)-M-1 was a weak antagonist at histamine H1 receptors (pKB = 6.49) [2]. After 12 weeks of sarpogrelate administration, FBF and LBF responses during RH showed significant increases from 13.2 +/- 1.7 to 18.1 +/- 2.2 mL/min per 100 mL tissue (P < 0.01) and from 8.2 +/- 0.9 to 14.2 +/- 2.1 mL/min per 100 mL tissue (P < 0.05), respectively. Sarpogrelate-induced augmentation of FBF and LBF responses to RH was maintained at 24 weeks. Long-term oral administration of sarpogrelate improves vascular function in patients with PAD [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 585.9ºC at 760 mmHg |
|---|---|
| Melting Point | 145-148°C |
| Molecular Formula | C24H32ClNO6 |
| Molecular Weight | 465.967 |
| Flash Point | 308.1ºC |
| Exact Mass | 465.191803 |
| PSA | 85.30000 |
| LogP | 3.99940 |
| Vapour Pressure | 1.43E-14mmHg at 25°C |
| Storage condition | Desiccate at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H400 |
| Precautionary Statements | P273 |
| Hazard Codes | Xn,N |
| Risk Phrases | 22-50/53 |
| Safety Phrases | 60-61 |
| RIDADR | UN 3077 9 / PGIII |
|
~% 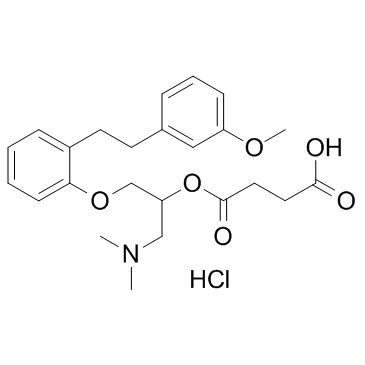
135159-51-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 33, # 6 p. 1818 - 1823 |
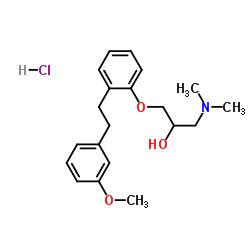
| Precursor 2 | |
|---|---|
| DownStream 2 | |

![(R,S)-1-[2-[2-(3-methoxyphenyl)ethyl]phenoxy]-3-(dimethylamino)-2-propanol structure](https://image.chemsrc.com/caspic/018/135963-42-7.png)