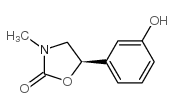
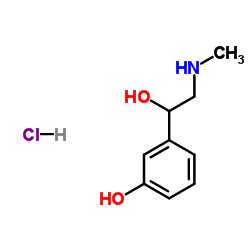
61-76-7
| Name | phenylephrine hydrochloride |
|---|---|
| Synonyms |
consdrin
(R)-3-Hydroxy-a-[(methylamino)methyl]benzenemethanol Hydrochloride (R)-(-)-Phenylephrine (hydrochloride) almefrin synasal phenylephrine HCl 3-[(1R)-1-Hydroxy-2-(methylamino)ethyl]phenol hydrochloride (1:1) 3-[(1R)-1-hydroxy-2-(méthylamino)éthyl]phénol chlorhydrate l-1-(m-Hydroxyphenyl)-2-methylaminoethanol Hydrochloride emagrin fenilfar AK-Dilate Benzenemethanol, 3-hydroxy-α-[(methylamino)methyl]-, (αR)-, hydrochloride (1:1) benzenemethanol, 3-hydroxy-α-[(methylamino)methyl]-, (αR)-, hydrochloride rhinall l-a-Hydroxy-b-methylamino-3-hydroxy-1-ethylbenzene Hydrochloride AK-Nefrin m-Sympatol Metaoxedrin Pyracort D Phenylephrine hydrochloride efricel Mezaton lexatol uri fenox EINECS 200-517-3 MFCD00012605 1-m-Hydroxy-a-[(methylamino)methyl]benzyl Alcohol Hydrochloride Isophrin 3-[(1R)-1-Hydroxy-2-(methylamino)ethyl]benzololhydrochlorid |
| Description | (R)-(-)-Phenylephrine hydrochloride is a selective α1-adrenoceptor agonist with pKis of 5.86, 4.87 and 4.70 for α1D, α1B and α1A receptors respectively. |
|---|---|
| Related Catalog | |
| Target |
pKi: 5.86 (α1D), 5.86 (α1B), 5.86 (α1A)[1] |
| In Vitro | (R)-(-)-Phenylephrine is a selective α1-adrenoceptor agonist with pKi values of 5.86, 4.87 and 4.70 for α1D, α1B and α1A receptors respectively[1][2]. Phenylephrine promotes cardiac fibroblast proliferation. Phenylephrine activates CaN and evokes NFAT3 nuclear translocation. It suggests that the Ca(2+)/CaN/NFAT pathway mediates phenylephrine -induced cardiac fibroblast proliferation, and this pathway might be a possible therapeutic target in cardiac fibrosis[3]. |
| In Vivo | Perfusion of hearts with 100 μM phenylephrine causes a rapid (maximal at 10 min) 12-fold activation of two p38-MAPK isoforms. α1-adrenoceptor agonists such as phenylephrine increase the contractility of the heart. Phenylephrine also activates SAPKs/JNKs in neonatal ventricular myocytes[4]. Phenylephrine could increase the alveolar fluid clearance in high tidal volume-ventilated rats and accelerate the absorption of pulmonary edema[5]. |
| Animal Admin | Rat: A total of 170 male Wistar rats are randomLy allocated into 17 groups (n=10) using random number tables. Short-term (40 minutes) mechanical ventilation with high tidal volume is performed to induce lung injury, impair active Na+ transport and lung liquid clearance in the rats. Unventilated rats serves as controls. To demonstrate the effect of phenylephrine on alveolar fluid clearance, phenylephrine at different concentrations (10, 1, 0.1, 0.01, and 0.001 μM) is injected into the alveolar space of the HVT ventilated rats[5]. |
| References |
| Boiling Point | 341.1ºC at 760 mmHg |
|---|---|
| Melting Point | 143-145 °C(lit.) |
| Molecular Formula | C9H14ClNO2 |
| Molecular Weight | 203.666 |
| Flash Point | 163.4ºC |
| Exact Mass | 203.071304 |
| PSA | 52.49000 |
| LogP | 1.83790 |
| Index of Refraction | -45.5 ° (C=1, H2O) |
| Water Solubility | >=10 g/100 mL at 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | DO7525000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2937229000 |
| Precursor 10 | |
|---|---|
| DownStream 2 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |



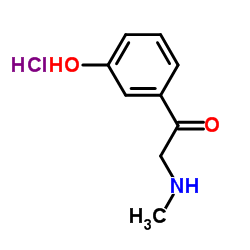
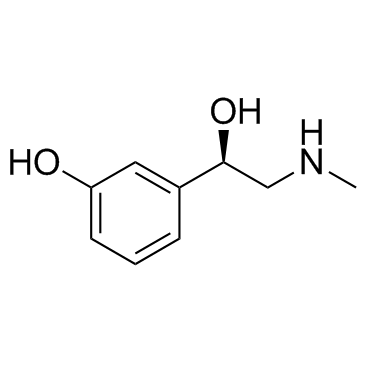
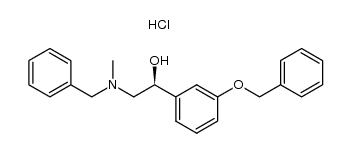


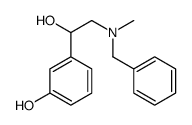
![1-(3-Hydroxyphenyl)-2-[methyl(phenylmethyl)amino]-ethanone hydrochloride structure](https://image.chemsrc.com/caspic/463/71786-67-9.png)
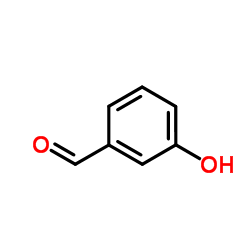
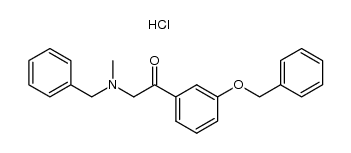
![3-[tert-butyl(dimethyl)silyl]oxybenzaldehyde structure](https://image.chemsrc.com/caspic/296/96013-95-5.png)