90417-38-2
| Name | nu 1025 |
|---|---|
| Synonyms |
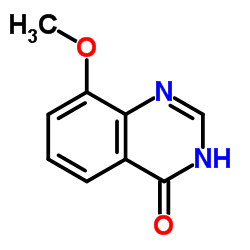
8-Hydroxy-2-methylquinazolin-4(1H)-one
2-methylquinazoline-4,8-diol MFCD00942555 4,8-quinazolinediol, 2-methyl- 8-hydroxy-2-methyl-1H-quinazolin-4-one 8-hydroxy-2-methylquinazolin-4(3H)-one 8-Hydroxy-2-methyl-4(1H)-quinazolinone 4(1H)-Quinazolinone, 8-hydroxy-2-methyl- 8-Hydroxy-2-methyl-4(3H)-quinazolinone |
| Description | NU1025 is a potent PARP inhibitor with an IC50 of 400 nM and a Ki of 48 nM. NU1025 potentiates the cytotoxicity of ionizing radiation and anticancer drugs. NU1025 has anti-cancer and neuroprotective activity[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 400 nM (PARP)[2] Ki: 48 nM (PARP)[3] |
| In Vitro | NU1025 (0.2 mM) pretreatment restores cell viability to approximately 73% and 82% in H2O2 and SIN-1 injured cells, respectively[1]. NU1025 enhances the cytotoxicity of the DNA-methylating agent MTIC, γ-irradiation and bleomycin 3.5-, 1.4- and 2-fold respectively in L1210 cells. The recovery from potentially lethal γ-irradiation damage cytotoxicity in plateau-phase cells is also inhibited by NU 1025. NU1025 causes a marked retardation of DNA repair[2]. Cell Viability Assay[1] Cell Line: PC12 cells Concentration: 0.2 mM Incubation Time: 6.5 hours Result: Restored cell viability to approximately 73% and 82% in H2O2 and SIN-1 injured cells. |
| In Vivo | NU1025 (1-3 mg/kg; intraperitoneal injection; male Sprague Dawley rats) treatment at 1 and 3 mg/kg reduces total infarct volume to 25% and 45%, respectively, when administered 1 h before reperfusion. NU1025 also produces significant improvement in neurological deficits. Neuroprotection with NU1025 is associated with reduction in PAR accumulation, reversal of brain NAD depletion and reduction in DNA fragmentation[1]. Animal Model: Male Sprague Dawley rats (250-270 g) induced focal cerebral ischemia[1] Dosage: 1 mg/kg, 3 mg/kg Administration: Intraperitoneal injection Result: At 1 and 3 mg/kg, reduced total infarct volume to 25% and 45%, respectively. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 345.4±44.0 °C at 760 mmHg |
| Melting Point | 253-258ºC |
| Molecular Formula | C9H8N2O2 |
| Molecular Weight | 176.172 |
| Flash Point | 162.7±28.4 °C |
| Exact Mass | 176.058578 |
| PSA | 65.98000 |
| LogP | 0.35 |
| Appearance | solid | off-white |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.678 |
| Storage condition | −20°C |
| Water Solubility | DMSO: 35 mg/mL, soluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | 22 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
|
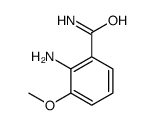
~66% 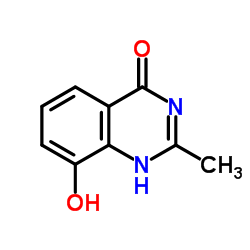
90417-38-2 |
| Literature: Newcastle University Ventures Limited Patent: US6156739 A1, 2000 ; |
|
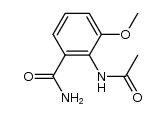
~% 
90417-38-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 26 p. 5247 - 5256 |
|
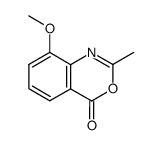
~% 
90417-38-2 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 26 p. 5247 - 5256 |
|
~% 
90417-38-2 |
| Literature: Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, , vol. 24, p. 1182 - 1184 |
|
~% 
90417-38-2 |
| Literature: Journal of Scientific and Industrial Research, , vol. 17 C, p. 193,194 |
|
~% 
90417-38-2 |
| Literature: WO2004/31161 A1, ; Page 117 ; |
| Precursor 6 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |