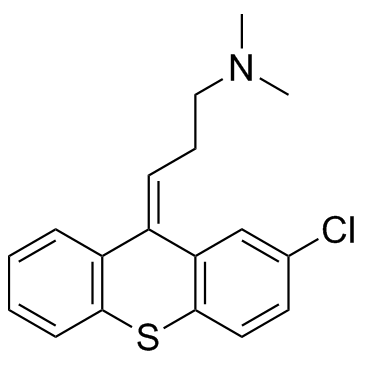
113-59-7
| Name | chlorprothixene |
|---|---|
| Synonyms |
1-Propanamine, 3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-, (3Z)-
Ro 4-0403 Tarasan (Z)-chlorprothixene Truxaletten Chlorprothixene (3Z)-3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethylpropan-1-amine chloroprothixene CPX N 714C (Z)-2-Chloro-9-(ω-dimethylaminopropylidene)thioxanthene (Z)-2-Chloro-9-(3'-dimethylaminopropylidene)thioxanthene Paxyl Truxil (Z)-2-Chloro-N,N-dimethylthioxanthene-D9,g-propylamine (Z)-a-2-Chloro-10-(3-dimethylaminopropylidene)thiaxanthene Taractan EINECS 204-032-8 Truxal 1-Propanamine, 3- (2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-, (Z)- 1-Propanamine, 3-(2-chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-, (Z)- (3Z)-3-(2-Chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-1-propanamine mk184 n714 |
| Description | Chlorprothixene has strong binding affinities to dopamine and histamine receptors, such as D1, D2, D3, D5, H1, 5-HT2, 5-HT6 and 5-HT7, with Ki of 18 nM, 2.96 nM, 4.56 nM, 9 nM, 3.75 nM, 9.4 nM, 3 nM and 5.6 nM, respectively.Target: Dopamine ReceptorChlorprothixene exerts strong binding affinities to the dopamine and histamine receptors, such as D1, D2, D3, D5 and H1 with Ki values of 18nM, 2.96 nM, 4.56 nM, 9 nM and 3.75 nM, respectively, but has little affinity to H3 (Ki >1000 nM) [1]. Chlorprothixene also shows high affinities for both rat 5-HT6 from stably transfected HEK-293 cells, and rat 5-HT7 receptors from transiently expressed COS-7 cells, with Ki values of 3 nM and 5.6 nM, respectively [2].Administration of Chlorprothixene restores normal ceramide concentrations in murine bronchial epithelial cells, reduces inflammation in the lungs of mice with cystic fibrosis (CF) and prevents infection with Pseudomonas aeruginosa, by inhibiting acidsphingomyelinase (Asm) and not neutral sphingomyelinase (Nsm) [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 435.0±45.0 °C at 760 mmHg |
| Melting Point | 97-98° |
| Molecular Formula | C18H18ClNS |
| Molecular Weight | 315.860 |
| Flash Point | 216.9±28.7 °C |
| Exact Mass | 315.084839 |
| PSA | 28.54000 |
| LogP | 6.05 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.683 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| HS Code | 2932999099 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |