131875-08-6
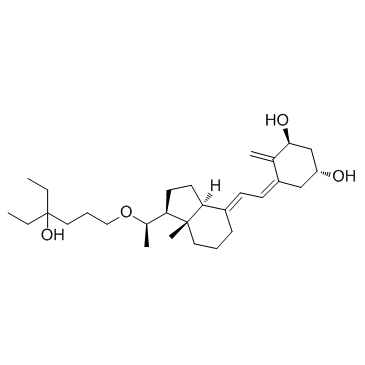
| Name | (1R,3S,5Z)-5-[(2E)-2-[(1S,3aS,7aS)-1-[(1R)-1-(4-ethyl-4-hydroxyhexoxy)ethyl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol |
|---|---|
| Synonyms |
(5Z,7E,20R)-20-((4-Ethyl-4-hydroxyhexyl)oxy)-9,10-secopregna-5,7,10(19)-triene-1a,3b-diol
LEVORPHANOL TARTRATE DIHYDRATE (1S-(1a(S*),3ab,4E(1S*,3R*,5Z),7aa))-5-((1-(1-((4-Ethyl-4-hydroxyhexyl)oxy)ethyl)octahydro-7a-methyl-4H-inden-4-ylidene)ethylidene)-4-methylene-1,3-cyclohexanediol LEVORPHANOL TARTRATE L-3-HYDROXY-N-METHYLMORPHINAN Lexacalcitol LEVORPHANOL TARTRATE SALT Levo-dromoran (tn) Levorphanol Tartrate CII (500 mg) Levorphanol Tartrate (CII),USP 1,3-Cyclohexanediol, 5-[(2E)-2-[(1S,3aS,7aS)-1-[(1R)-1-[(4-ethyl-4-hydroxyhexyl)oxy]ethyl]octahydro-7a-methyl-4H-inden-4-ylidene]ethylidene]-4-methylene-, (1R,3S,5Z)- (1S,3R,5Z,7E,9ξ,20R)-20-[(4-Ethyl-4-hydroxyhexyl)oxy]-9,10-secopregna-5,7,10-triene-1,3-diol 20-epi-22-oxa-24a,26a,27a-tri-homo-1,25-dihydoxyvitamin D3 (1S,3R,5Z,7E,9ξ,20R)-20-[(4-Ethyl-4-hydroxyhexyl)oxy]-9,10-secopregna-5,7,10-trien-1,3-diol UNII:9G3DCA3958 levorphanol tartrate methanol solution LEVORPHANOLTARTRATE,USP |
| Description | Lexacalcitol (KH1060), a vitamin D analog, is potent regulators of cell growth and immune responses. Lexacalcitol can be used for the research of graft rejection, psoriasis, cancer and auto-immune diseases[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Lexacalcitol inhibits cell proliferation by 50% at 10-12 M (14,000 times more active than la,25(OH)2D3) in human histiocytic lymphoma cell line U 937[1]. Lexacalcitol inhibits interleukin-1-induced mouse thymocyte proliferation by 50% at 3×10-16 M , allogeneic stimulation of mouse spleen lymphocytes at 5×10-15 M[1]. |
| In Vivo | Lexacalcitol (0.5 mg/kg/2 days; i.p.) in combination with cyclosporin A (CyA) can prevent autoimmune destruction of syngeneic islet grafts in spontaneously diabetic NOD recipients[2]. Animal Model: Male and female spontaneously diabetic NOD mice[2] Dosage: 0.5 mg/kg/2 days Administration: Intraperitoneal injection Result: Single treatment with KH1060 or CyA did not result in statistically significant suppression of early graft failure, while the combination of KH1060 and CyA can prevent early graft failure and delay graft rejection of xenogeneic islets in spontaneously diabetic NOD mice. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 600.3±55.0 °C at 760 mmHg |
| Molecular Formula | C29H48O4 |
| Molecular Weight | 460.689 |
| Flash Point | 316.8±31.5 °C |
| Exact Mass | 460.355255 |
| PSA | 69.92000 |
| LogP | 5.62 |
| Vapour Pressure | 0.0±3.9 mmHg at 25°C |
| Index of Refraction | 1.542 |
| Storage condition | 2-8℃ |
|
~% 
131875-08-6 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 9, # 2 p. 525 - 535 |
|
~% 
131875-08-6 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 9, # 2 p. 525 - 535 |
|
~% 
131875-08-6 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 9, # 2 p. 525 - 535 |
|
~% 
131875-08-6 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 9, # 2 p. 525 - 535 |
|
~% 
131875-08-6 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 9, # 2 p. 525 - 535 |
|
~% 
131875-08-6 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 9, # 2 p. 525 - 535 |
|
~% 
131875-08-6 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 9, # 2 p. 525 - 535 |
|
~% 
131875-08-6 |
| Literature: Bioorganic and Medicinal Chemistry, , vol. 9, # 2 p. 525 - 535 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |