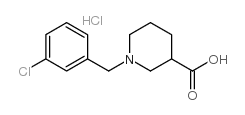
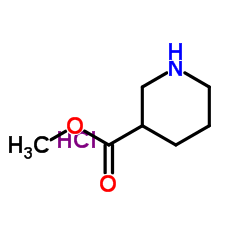
498-95-3
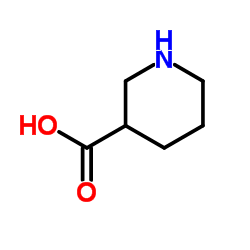
| Name | nipecotic acid |
|---|---|
| Synonyms |
hexahydronicotinic acid
MFCD00005992 (±)-Nipecotic acid (±)-Piperidine-3-carboxylic acid Nipecotic acid UNII:1U1QTN40SY Piperidine-3-carboxylic acid EINECS 207-873-9 (±)-3-Piperidine carboxylic acid 3-Piperidinecarboxylic acid H-DL-Nip-OH |
| Description | Nipecotic acid ((±)-β-Homoproline) is a potent inhibitor of neuronal and glial-aminobutyric acid (GABA) uptake in vitro. Nipecotic acid can also directly activate GABAA-like chloride channels, with an EC50 of approximately 300 μM[1][2]. |
|---|---|
| Related Catalog | |
| Target |
GABA Receptor[1] |
| In Vitro | Nipecotic acid (1 mM) activated inward unitary currents when applied to outside-out patches of paraventricular neurones[1]. |
| In Vivo | Nipecotic acid does not readily cross the blood-brain barrier (BBB)[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 265.8±33.0 °C at 760 mmHg |
| Melting Point | 261ºC (dec.) |
| Molecular Formula | C6H11NO2 |
| Molecular Weight | 129.157 |
| Flash Point | 114.5±25.4 °C |
| Exact Mass | 129.078979 |
| PSA | 49.33000 |
| LogP | -0.04 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.479 |
| Storage condition | Store at 0-5°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TM6125380 |
| HS Code | 2933399090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |





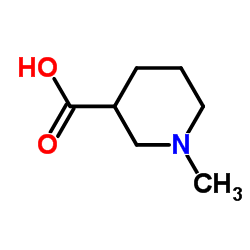

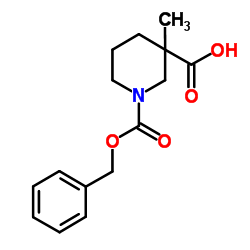
![1-[(benzyloxy)carbonyl]-3-methylpiperidine-3-carboxylic acid structure](https://image.chemsrc.com/caspic/190/174543-78-3.png)