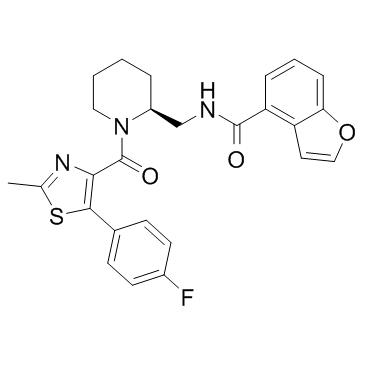
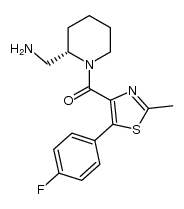
| Description |
SB-649868 is a potent and selective orally active orexin (OX) 1 and OX2 receptor antagonist (pKi =9.4 and 9.5 at the OX1 and OX2 receptor, respectively).
|
| Related Catalog |
|
| Target |
pKi: 9.4 (OX1), 9.5 (OX2)[1]
|
| In Vitro |
SB-649868 is identified as one the most in vitro potent dual OX1 and OX2 receptor antagonist known at that time (pKi=9.4 and 9.5 at the OX1 and OX2 receptor, respectively) [1]. SB-649868 antagonizes orexin-A-induced inositol 1 phosphate (IP1) accumulation with the following pKB value (OX1=9.67; OX2=9.64). SB-649868 displaces the [3H]ACT-078573 receptor binding with the following pKi values: OX1=9.27; OX2=8.91. Increasing concentrations of SB-649868 (0.3 nM-30 nM) induces a rightward shift of the orexin-A CRCs with a depression of the agonist efficacy suggesting a clear non-surmountable behavior. The calculated apparent pKb values are 9.67±0.03 and 9.64±0.07 for OX1 and OX2[2].
|
| In Vivo |
Pharmacokinetic studies in the male CD rat, performed at 1 mg/kg, iv and 3 mg/kg, po, demonstrate an excellent pharmacokinetic profile for a hypnotic agent featuring moderate clearance in plasma (Clp=24 mL/min/kg), short half-life of (<0.6 h) and a low volume of distribution (Vss=1.1 l/kg), coupled with excellent oral bioavailability (F=85%) and good exposure in plasma (Cmax=333 ng/mL). A brain to blood ratio (B/B) of 0.1:1 is observed 1 h after iv administration, a value in line with the expected partition between the two compartments based on the lower tissue binding observed in vitro in brain tissues (fraction unbound/brain=5.28%) with respect to plasma proteins (fraction unbound/plasma=1.34%). SB-649868, administered orally 3 h before OX-A injection at doses of 1, 3 and 10 mg/kg, causes a dose-dependent reduction of OX-A induced grooming as measured by total time spent grooming and number of grooming bouts (p <0.01 at 3 and 10 mg/kg po) [1]. From dissociation kinetic studies using [3H]ACT-078573, the calculated long half-life, (t1/2) supports the non-surmountability profile of SB-649868 (t1/2=35.91 min) at OX1 orexin receptor. The long or moderately long t1/2values for SB-649868 at OX2 orexin receptor (t1/2=8.09 min)[2].
|
| Cell Assay |
Chinese Hamster Ovary (CHO) cells stably transfected with human OX1 orexin receptor are cultured in Dulbecco's modified Eagle's medium F12 Ham, supplemented with 10% fetal bovine serum (FBS), 2 mg/mL glutamine, 600 μg/ml geneticin at 37 °C in an atmosphere of 95% air and 5% CO2. CHO cells stably transfected with human OX2 orexin receptor are cultured in alpha-MEM supplemented with 10% FBS, 100 units/mL penicillin G, 100 units/mL streptomycin and 400 μg/mL geneticin, at 37 °C in an atmosphere of 95% air and 5% CO2. Accumulation of IP1 is measured using IP-One HTRF terbium cryptate-based assay. OX1-CHO cells are seeded into white 384-well plate at the cell density of 1×104 cells per well and cultured for 24 h in the presence of 5 mM sodium butyrate while OX2-CHO cells are seeded at the cell density of 4×104 cells per well and cultured for 24 h in culture medium. After washings Hank's Balanced Salt Solution (HBSS) at room temperature containing 20 mM HEPES pH 7.4, 50 mM, LiCl and 0.1% Bovine Serum Albumin (BSA) cells are pre-incubated for 45 min with antagonist and then treated with agonist for 60 min at 37 °C. Detection reagents, IP1-d2 tracer and anti-IP1-cryptate are diluted in lysis buffer and added to the cells. Following 60 min incubation at room temperature, time-resolved fluorescence at 615 nm and 665 nm are measured with Envision Multilabel flash lamp reader with 100 flashes and 400 μs integration time[2].
|
| References |
[1]. Di Fabio R, et al. Discovery process and pharmacological characterization of a novel dual orexin 1 and orexin 2receptor antagonist useful for treatment of sleep disorders. Bioorg Med Chem Lett. 2011 Sep 15;21(18):5562-7. [2]. Faedo S, et al. Functional and binding kinetic studies make a distinction between OX1 and OX2 orexin receptorantagonists. Eur J Pharmacol. 2012 Oct 5;692(1-3):1-9.
|