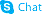540769-28-6
| Name | 1-[2-(4-benzyl-4-hydroxypiperidin-1-yl)ethyl]-3-(2-methylquinolin-4-yl)urea |
|---|---|
| Synonyms |
UNII-ULD9ZKE457
Palosuran ACT 058362 Urea, N-[2-[4-hydroxy-4-(phenylMethyl)-1-piperidinyl]ethyl]-N'-(2-Methyl-4-quinolinyl)- |
| Description | Palosuran (ACT-058362) is a new potent and specific antagonist of the human UT receptor with an IC50 of 3.6±0.2 nM. IC50 Value: 3.6±0.2 nM [1]Target: Urotensin Receptor (GPR14)in vitro: Palosuran inhibited 125I-U-II binding to human UT receptors in membrane preparations from CHO cells carrying the human UT receptors almost as potently as cold U-II, with an IC50 of 3.6 ± 0.2 nM. On cells, the inhibitory binding potency of palosuran against human UT receptor was lower than on membranes (IC50 = 46.2 ± 13 nM on TE 671 cells and 86 ± 30 nM on recombinant CHO cells). Compared with the human UT receptor, the binding inhibitory potency of palosuran against the rat UT receptor was lower in membrane preparation (400-fold), as well as in cells (>120-fold) [1].in vivo: Long-term treatment of streptozotocin-induced diabetic rats with palosuran improved survival, increased insulin, and slowed the increase in glycemia, glycosylated hemoglobin, and serum lipids. Furthermore, palosuran increased renal blood flow and delayed the development of proteinuria and renal damage [2]. Palosuran was rapidly absorbed with maximum plasma concentrations at 1 hour after drug administration. The accumulation factor was 1.7 (geometric mean) (95% confidence interval, 1.3 to 2.1).Palosuran was well tolerated [3]. In mesenteric vessels, palosuran treatment up-regulated expression of RhoA and Rho-kinase, increased Rho-kinase-activity, and diminished nitric oxide (NO)/cyclic guanosine 3',5'-monophosphate (cGMP) signaling. Moreover, palosuran increased renal blood flow, sodium, and water excretion in BDL rats [4].Toxicity: Palosuran was well tolerated. No serious adverse events or dose-related adverse events were reported. No treatment-related pattern was detected for vital signs, clinical laboratory parameters, or electrocardiography parameters [5]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C25H30N4O2 |
|---|---|
| Molecular Weight | 418.53100 |
| Exact Mass | 418.23700 |
| PSA | 84.21000 |
| LogP | 3.29850 |
| Appearance | white solid |
| Storage condition | -20℃ |