866933-46-2
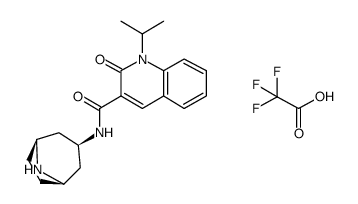
| Name | N-[8-[(2R)-2-hydroxy-3-[methyl(methylsulfonyl)amino]propyl]-8-azabicyclo[3.2.1]octan-3-yl]-2-oxo-1-propan-2-ylquinoline-3-carboxamide |
|---|
| Description | Velusetrag (TD-5108) is an orally active, potent and selective agonist of serotonin 5-HT4 receptor (5-HT4R), with a pKi of 7.7. Velusetrag exhibits no affinity (Ki>10 μM) for 5-HT2A and 5-HT2B receptors. Velusetrag can be used for the research of gastrointestinal diseases and Parkinson's disease[1][2][3][4][5]. |
|---|---|
| Related Catalog | |
| Target |
5-HT4 Receptor:7.7 (pKi) |
| In Vitro | Velusetrag (10 pM-100 μM) concentration-dependently increases the cAMP in HEK-293 cells stably transfected with the h5-HT4(c) receptor, with a pEC50 of 8.3[1]. Velusetrag (100 pM-1 μM) produces concentration-dependent contraction of the guinea pig colonic longitudinal muscle/myenteric plexus (LMMP), with a pEC50 of 7.9[1]. Velusetrag (0.001-10 μM) produces a concentration-dependent relaxation of the carbachol (3 μM)-precontracted rat esophagus, with a pEC50 of 7.9[1]. |
| In Vivo | Velusetrag (3 mg/kg; a single i.p.) significantly improves the facilitation of contextual fear extinction in PD mice[3]. Velusetrag (3 mg/kg; a single i.p.) increases hippocampal cAMP levels in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice[3]. Velusetrag (0.003-3 mg/kg; a single s.c.) increases colonic transit in a dose-dependent manner and reduces the time taken for excretion of the dye in guinea pigs[2]. Velusetrag (0.003-1 mg/kg; a single i.v.) dose-dependently increases inter-crystal distance, consistent with relaxation of the oesophagus in rats[2]. Animal Model: Male C57BL/6 mice (7-8 weeks old) were injected with MPTP[3] Dosage: 3 mg/kg Administration: A single i.p. Result: Improved facilitation of contextual fear extinction. Did not improve the impaired rotarod performance in PD mice. |
| References |
| Density | 1.34g/cm3 |
|---|---|
| Molecular Formula | C25H36N4O5S |
| Molecular Weight | 504.64200 |
| Exact Mass | 504.24100 |
| PSA | 123.82000 |
| LogP | 3.15340 |
|
~% 
866933-46-2 |
| Literature: US2008/287486 A1, ; Page/Page column 6 ; |
|
~% 
866933-46-2 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 22, # 19 p. 6048 - 6052 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |