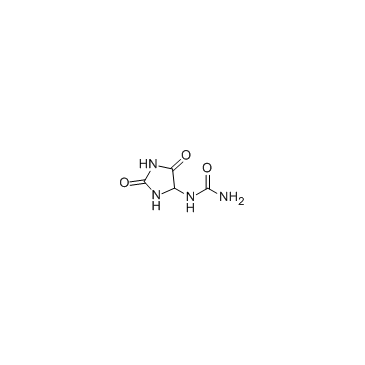
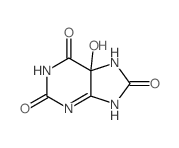
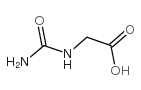
97-59-6
| Name | allantoin |
|---|---|
| Synonyms |
Allantol
DL-Allantoin uniderma ALL Allantoin Septalan N-(2,5-Dioxo-4-imidazolidinyl)urea 2,5-Dioxo-4-imidazolidinyl-urea Urea, (2,5-dioxo-4-imidazolidinyl)- (9CI) 4-ureido-2,5-Imidazolidinedione toin 5-ureidohydantoin ALANTOIN (±)-Allantoin Sebical 4H-imidazole-2,5-diol, 4-[(hydroxyiminomethyl)amino]- Urea, (2,5-dioxo-4-imidazolidinyl)- Alantan 1-(2,5-Dioxoimidazolidin-4-yl)harnstoff Allantoin (8CI) 1-(2,5-dioxoimidazolidin-4-yl)urea 2,5-dioxo-4-imidazolidinyl urea 1-(2,5-Dioxo-4-imidazolidinyl)urea Urea, N-(2,5-dioxo-4-imidazolidinyl)- MFCD00005260 Psoralon 5-Ureido-2,4-imidazolidindion 5-ureido-Hydantoin EINECS 202-592-8 |
| Description | Allantoin is a skin conditioning agent that promotes healthy skin, stimulates new and healthy tissue growth. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Allantoin is a well-known cosmetic ingredient reported to have anti-oxidative and anti-inflammatory activities[1]. Allantoin attenuates apoptosis and cytotoxicity and increased the viability of STZ-induced β-cells in a dose-dependent manner. Allantoin decreases the level of caspase-3 and increases the level of phosphorylated B-cell lymphoma 2 (Bcl-2) expression. Allantoin has been demonstrated to activate imidazoline 3 (I3) receptors[2]. |
| In Vivo | The subchronic administration of allantoin (1, 3 or 10 mg/kg, for 7 days) significantly increases the latency time measured during the passive avoidance task in scopolamine-induced cholinergic blockade and normal naive mice. Allantoin treatment (3 or 10 mg/kg, for 7 days) also increases the expression levels of phosphorylated phosphatidylinositide 3-kinase (PI3K), phosphorylated protein kinase B (Akt) and phosphorylated glycogen synthase kinase-3β (GSK-3β). Allantoin significantly increases the neuronal cell proliferation of immature neurons in the hippocampal dentate gyrus region[1]. Daily injection of allantoin for 8 days in STZ-treated rats significantly lowers plasma glucose and increases plasma insulin levels [2]. Allantoin decreases blood pressures in SHRs at 30 minutes, as the most effective time. Also, this antihypertensive action is shown in a dose-dependent manner from SHRs treated with allantoin. Moreover, in anesthetized rats, allantoin inhibits cardiac contractility and heart rate. Also, the peripheral blood flow is markedly increased by allantoin[3]. |
| Cell Assay | Pancreatic β-cells are treated with 1, 10, 100 μM of allantoin before 30 min prior to the addition of 5 mM STZ and incubated for 6 h. Cell viability is measured using the ApoTox-Glo triplex assay[2]. |
| Animal Admin | Rats: Animals are randomly assigned into four groups: (I) the control group treated with the vehicle, saline; (II) the allantoin group treated by intravenous injection of allantoin at 0.5 mg/kg; (III) the allantoin+efaroxan group treated with allantoin at the most effective dose (0.5 mg/kg, i.v.) and efaroxan at effective dose (1.5 mg/kg, i.v.) 30 minutes before injection of allantoin; and (IV) the allantoin treated SHRs group treated by intravenous injection of allantoin at various dose for desired time. After treatment of allantoin, the rats are placed into a holder for the determination of the mean blood pressure[3]. Mice: For memory ameliorating study, mice are administered vehicle solution, allantoin (1, 3 or 10 mg/kg, p.o.) or donepezil (5 mg/kg, p.o.) at the same time (10:00-12:00 a.m) and same place for 7 days. For memory enhancing study, mice are administered vehicle solution, allantoin (1, 3 or 10 mg/kg, p.o.) or piracetam (200 mg/kg, i.p.). The final administration of allantoin, donepezil or piracetam is performed 1 h before an acquisition trial in the passive avoidance task[1]. |
| References |
[3]. Chen MF, et al. Antihypertensive action of allantoin in animals. Biomed Res Int. 2014;2014:690135. |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 478ºC |
| Melting Point | 230 °C (dec.)(lit.) |
| Molecular Formula | C4H6N4O3 |
| Molecular Weight | 158.115 |
| Flash Point | 230-234°C |
| Exact Mass | 158.043991 |
| PSA | 113.32000 |
| LogP | -2.89 |
| Index of Refraction | 1.616 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn,Xi |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 1 |
| RTECS | YT1600000 |
| HS Code | 2933790090 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |