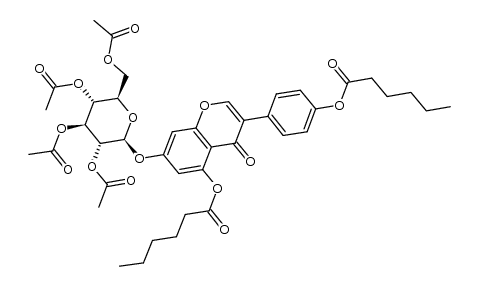
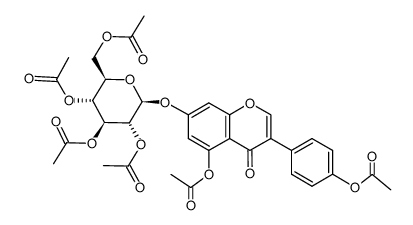
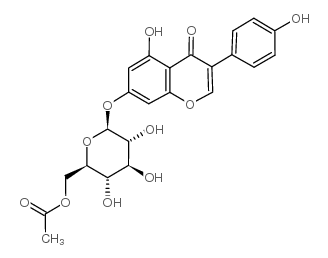
529-59-9
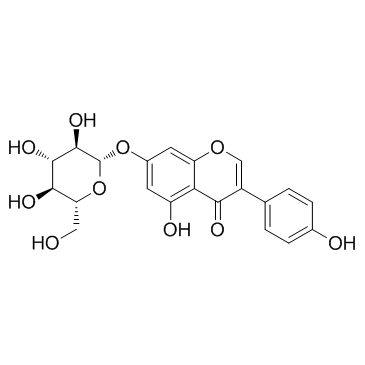
| Name | genistein 7-O-β-D-glucoside |
|---|---|
| Synonyms |
7-(b-D-Glucopyranosyloxy)-5-hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one
5-Hydroxy-3-(4-hydroxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-on Genisteol 7-monoglucoside Genistin 5-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl-β-D-glucopyranoside 4H-1-Benzopyran-4-one, 7-(β-D-glucopyranosyloxy)-5-hydroxy-3-(4-hydroxyphenyl)- 4',5,7-trihydroxyisoflavone-7-D-glucoside Diadzin MFCD00016883 4',5,7-Trihydroxyisoflavone 7-glucoside GLUCOSYL-7-GENISTEIN Genistein-7-glucoside genistein 7-O-beta-D-glucoside Genistein 7-O-β-D-glucoside Genistein, 7-O-β-D-glucoside genistein 7-O-glucoside 5-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl β-D-glucopyranoside Genistein 7-O-b-D-Glucoside Soybean Extract 5-Hydroxy-3-(4-hydroxyphényl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]oxy}-4H-chromén-4-one Genistein-7-O-β-D-glucopyranoside Genistine Genistein glucoside Genistoside Genistein, 7-β-D-glucopyranoside 5-Hydroxy-3-(4-hydroxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one 4H-1-Benzopyran-4-one, 7- (β-D-glucopyranosyloxy)-5-hydroxy-3-(4-hydroxyphenyl)- Genistein-7-O-glucoside Genistein 7-glucoside |
| Description | Genistin is the major isoflavonoid of soybeans and soy products. |
|---|---|
| Related Catalog | |
| In Vitro | Genistin is the major isoflavonoid of soybeans and soy products. Genistin shows a dose-dependent superoxide scavenging effect and exhibits major effect at 200 μM, corresponding in activity to 0.08 U/mg protein superoxide dismutase (SOD). Results demonstrate that Genistin exhibits a significantly (P<0.01) and a dose-dependent inhibitory effect on the human cancer cell examined, and at higher concentration (100 μM), the cell viability is 59%. Genistin also induces a significant and dose-dependent increase in ROS formation when compare with the untreated control[1]. |
| In Vivo | Myocardial infarct is markedly diminished by pretreatment with Genistin, particularly at the high dose. After 1 h of reperfusion, preconditioning with Genistin at dosages of 20 to 60 mg/kg significantly attenuats the release of lactate dehydrogenase (LDH), creatine kinase (CK) in a dose-dependent manner compare with the I/R group. Results show that the level of malondialdehyde (MDA) is decreased and the activities of superoxide dismutase (SOD) and catalase (CAT) are increased as well as an increased glutathione (GSH) level in a dose-dependent manner by Genistin treatment in I/R. Pretreatment with Genistin (20, 40 and 60 mg/kg) also prevents the expression of P2X7, p-IκBα, and p-NF-κB p65 compare with the model group[2]. |
| Cell Assay | M14 human melanoma cells are used and grown in RPMI containing 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 25 μg/mL fungizone. After 24 h of incubation at 37°C under a humidified 5% carbon dioxide to allow cell attachment, the cells are treated with different concentrations (12, 25, 50, and 100 μM) of Genistin and daidzin, and incubated for 72 h under the same conditions[1]. |
| Animal Admin | Sprague-Dawley rats (male, 250 to 300 g) are used to establish the I/R injury animal model and used in this experiment. Rats are randomly apportioned in equal animals (n=10) to five experimental groups: (1) sham group: rats are subjected to the entire surgical procedure but without the induction of I/R; (2) model group: I/R injury animal model is constructed by left anterior descending coronary artery (LAD) ligation for 30 min, and then the LAD is allowed 1 h reperfusion; and (3) three Genistin-treated groups: different doses (20, 40, and 60 mg/kg body weight, resp.) of Genistin dissolved in 0.5% sodium carboxyl methyl cellulose (CMC-Na) solution are given intragastrically for 5 days before operation[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 788.9±60.0 °C at 760 mmHg |
| Melting Point | 254ºC |
| Molecular Formula | C21H20O10 |
| Molecular Weight | 432.378 |
| Flash Point | 280.7±26.4 °C |
| Exact Mass | 432.105652 |
| PSA | 170.05000 |
| LogP | 0.79 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.717 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DJ3093000 |
|
~% 
529-59-9 |
| Literature: European Journal of Organic Chemistry, , # 33 p. 5622 - 5629 |
|
~% 
529-59-9 |
| Literature: European Journal of Organic Chemistry, , # 33 p. 5622 - 5629 |
|
~% 
529-59-9 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 40, # 7 p. 1978 - 1980 |
|
~% 
529-59-9 |
| Literature: Agricultural and Biological Chemistry, , vol. 44, # 2 p. 469 - 470 |
|
~% 
529-59-9 |
| Literature: Chemische Berichte, , vol. 76, p. 1110 |
|
~% 
529-59-9 |
| Literature: Journal of Agricultural and Food Chemistry, , vol. 55, # 9 p. 3408 - 3413 |
| Precursor 5 | |
|---|---|
| DownStream 3 | |