1025720-94-8
| Name | N-{4-[(2-Amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl}-4-ethoxy -1-(4-fluorophenyl)-2-oxo-1,2-dihydro-3-pyridinecarboxamide |
|---|---|
| Synonyms |
3-Pyridinecarboxamide, N-[4-[(2-amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-1,2-dihydro-2-oxo-
Alimta (S)-2-[4-[2-(4-amino-2-oxo-3,5,7-triazabicyclo[4.3.0]nona-1(6),3,8-trien-9-yl)ethyl]benzoyl]aminopentanedioic acid Pemetrexed (INN) LYA N-(4-(2-Amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (S)-2-(4-(2-(2-Amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioic acid 1juj N-(4-(2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-D]pyrimidin-5-yl)ethyl)benzoyl)-L-glutamic acid N-{4-[(2-Amino-3-chloro-4-pyridinyl)oxy]-3-fluorophenyl}-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydro-3-pyridinecarboxamide Pemetrexed acid BMS777607 BMS 777607 |
| Description | BMS 777607 is a Met-related inhibitor for c-Met, Axl, Ron and Tyro3 with IC50s of 3.9 nM, 1.1 nM, 1.8 nM and 4.3 nM, respectively, and 40-fold more selective for Met-related targets than Lck, VEGFR-2, and TrkA/B, with more than 500-fold greater selectivity versus all other receptor and non receptor kinases. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3.9 nM (c-Met), 1.1 nM (Axl), 1.8 nM (Ron), 4.3 nM (Tyro3) |
| In Vitro | BMS 777607 is a selective ATP-competitive Met kinase inhibitor which potently blocks the autophosphorylation of c-Met with IC50 of 20 nM in GTL-16 cell lysates, and demonstrates selective inhibition of proliferation in Met-driven tumor cell lines, such as GTL-16 cell line, H1993 and U87[1]. BMS 777607 inhibits hepatocyte growth factor (HGF)-triggered c-Met autophosphorylation with IC50 of < 1 nM in PC-3 and DU145 prostate cancer cells. BMS 777607 has little effect on tumor cell growth, but exhibits inhibitory effect on HGF-induced cell scattering in PC-3 and DU145 cells, with almost complete inhibition at 0.5 μM. BMS 777607 also suppresses stimulated cell migration and invasion in a dose-dependent fashion (IC50 < 0.1 μM) in both cell lines[2]. Application of BMS 777607 (appr 10 μM) to the highly metastatic murine KHT cells for 2 hours potently eliminates basal levels of autophosphorylated c-Met with IC50 of 10 nM without affecting the total c-Met, leading to dose-dependent inhibition of phosphorylation of downstream signaling molecules including ERK, Akt, p70S6K and S6. Treatment with BMS 777607 (appr 1 μM) for 24 hours potently inhibits the KHT cell scatter, motility and invasion at doses in the nanomolar range which consists with MET gene knockdown, and modestly affects cell proliferation and colony formation[3]. |
| In Vivo | Oral administration of BMS 777607 (6.25-50 mg/kg) significantly reduces tumor volumes of the GTL-16 human tumor xenografts in athymic mice with no observed toxicity[1]. Administration of BMS 777607 (25 mg/kg/day) decreases the number of KHT lung tumor nodules (28.3%), improves the morphological hemorrhage, and significantly impairs the metastatic phenotype in the 6-8 week-old female C3H/HeJ mice injected with rodent fibrosarcoma KHT cells without apparent systemic toxicity compared to the control treatment. A low dose of BMS 777607 (10 mg/kg) also offers a mild but not significant inhibition of lung nodule formation compared to the vehicle control[3]. |
| Cell Assay | KHT cells are exposed to serial dilution of BMS 777607 for 96 hours, then the MTT assay and trypan blue exclusion are used for the determination of cell proliferation and cell death, respectively. KHT cell colonies are incubated with BMS 777607 for 24 hours and then stained with crystal violet (0.1%) and photographed for the assessment of cell scattering. 2 mm scratch on the confluent KHT cell monolayer is made using a sterilized 1 mL pipette tip followed by treated with BMS 777607 for 24 hours, then the number of cells that have migrated into the denuded area is counted on 4 random fields for the evaluation of cell migration. For the examination of cell invasion, the commercial transwell inserts (8 μM pore membrane) pre-loaded with Matrigel are incubated with serum-free medium in the presence or absence of BMS 777607 at 37°C for 2 hours to allow rehydration of Matrigel. Then cells suspended in serum-free medium are loaded onto the top chamber (5×103/insert) and complete medium (containing 10% FBS) is used in the lower chamber as a chemoattractant. After incubation for 24 hours, the Matrigel is removed and the inserts are stained with crystal violet. Invaded cells on the underside of the filter are photographed and counted. |
| Animal Admin | The pharemacokinetics of BMS 777607 are characterized in male Balb/C mice. Two groups of animals (N=6 per group, 20-25 g) are fasted overnight and receive BMS 777607 either as an intravenous (IV) bolus dose (5 mg/kg) via the tail vein or by gavage (10 mg/kg). The mice are fed 6 h after dosing. Blood samples (appr 0.2 mL) are obtained by retro-orbital bleeding at 0.05 (or 0.25 for oral), 0.5, 1, 3, 6, 8 and 24 h post dose. Within each group, half of the animals are bled at 0.05 (or 0.25 for oral), 1, 6 and 24 h, the other half are bled at 0.5, 3, and 8 h, resulting in a composite pharmacokinetic profile (3 mice per time point). Blood samples are allowed to coagulate and centrifuged at 4°C (1500-2000 ×g) to obtain serum. Serum samples are stored at appr 20°C until analysis by LC/MS/MS. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 667.9±55.0 °C at 760 mmHg |
| Molecular Formula | C25H19ClF2N4O4 |
| Molecular Weight | 512.893 |
| Flash Point | 357.7±31.5 °C |
| Exact Mass | 512.106262 |
| PSA | 112.69000 |
| LogP | 4.86 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.672 |
| Storage condition | -20℃ |
|
~75% 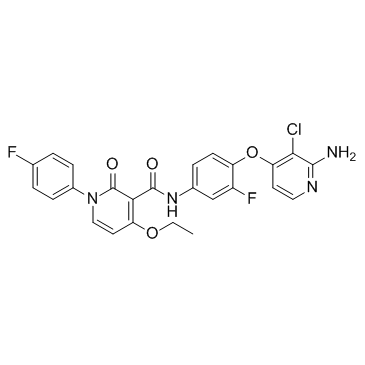
1025720-94-8 |
| Literature: US2008/114033 A1, ; Page/Page column 9 ; US 20080114033 A1 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
