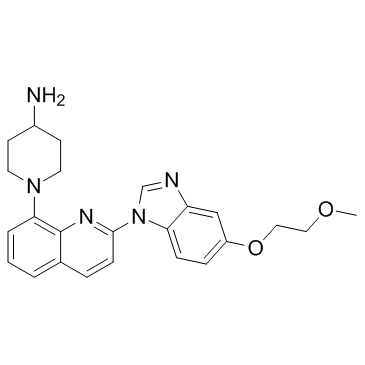
343787-29-1
| Name | 1-[2-[5-(2-methoxyethoxy)benzimidazol-1-yl]quinolin-8-yl]piperidin-4-amine |
|---|---|
| Synonyms |
1-[2-[5-(2-Methoxyethoxy)benzimidazol-1-yl]quinolin-8-yl]piperidin-4-ylamine
CS-0207 1-(2-(5-(2-methoxyethoxy)-1H-benzo[d]imidazol-1-yl)quinolin-8-yl)piperidin-4-amine 1-{2-[5-(2-Methoxyethoxy)-1H-benzimidazol-1-yl]-8-quinolinyl}-4-piperidinamine cc-529 4-Piperidinamine, 1-[2-[5-(2-methoxyethoxy)-1H-benzimidazol-1-yl]-8-quinolinyl]- UNII-0AM0WWD90A S1536_Selleck CP673451 CP-673451 |
| Description | CP-673451 is a potent and selective inhibitor of PDGFR with IC50s of 10 and 1 nM for PDGFRα and PDGFRβ, respectively. |
|---|---|
| Related Catalog | |
| Target |
PDGFRα:10 nM (IC50) PDGFRβ:1 nM (IC50) |
| In Vitro | CP-673451 efficiently suppresses the PDGFR downstream signaling pathway. It inhibits phosphorylation of Akt, GSK-3β, p70S6, and S6 in A549 cells in a concentration-dependent manner. CP-673451 (0.0625-4 μM) significantly reduces the viability of NSCLC cell lines A549 and H1299 in a time- and concentration-dependent manner, with IC50s of 0.49 and 0.61 μM, respectively. CP-673451 (1, 4 μM) induces apoptosis in non-small-cell lung cancer cells. CP-673451 (25, 100, or 400 nM) is effective at inhibiting migration and invasion of NSCLC cells by suppression of lamellipodia formation[1]. CP-673451 and crenolanib show selective lethality toward cells with CA. U2OS cells treated with 1 to 4 μM CP-673451 or crenolanib show a ruffled cell surface as a sign for alterations of the cortical actin cytoskeleton. CP-673451 attenuates PDGF-BB-induced signaling, and significantly enhances the phosphorylation of PDGFR-β downstream effectors, Akt and MEK[2]. CP-673,451 (0.5 μM) regulates cell proliferation through mechanisms involving reduced phosphorylation of GSK-3α and GSK-3β. CP-673,451 impairs rhabdosphere-forming capacity in both RD and RUCH2 cultures[3]. CP-673,451 inhibits PDGFR-β in PAE-β cells with an IC50 value of 6.4 nM. Besides, CP-673,451 incubation in H526 and PAE-β cells results in an IC50 value of 1.1 μM against c-kit[4]. |
| In Vivo | CP-673451 (20 mg/kg) leads to a medium suppression of tumor growth, while high-dose CP-673451 (40 mg/kg) strongly inhibits tumor growth in mice without significant weitht loss[1]. CP-673,451 (10, 33, and 100 mg/kg, p.o., b.i.d) inhibits the growth of Colo205 tumor in a dose-dependent manner, and similar tumor growth inhibition experiments completes on LS174T, H460, and U87MG xenografts, with no signs of morbidity or weight loss[4]. |
| Cell Assay | Cell proliferation/viability is analyzed using the CyQuant proliferation assay. Pre-starved cells are treated every 24 hours with vehicle (dimethyl sulfoxide) or 0.5 μM CP-673,451 diluted in serum-reduced medium (1.5 % FBS) for 96 hours. The amount of nucleic acid present in lysed cells is normalized to the amount when treatment is initiated. Cell proliferation/viability in response to 300 ng/mL PDGF-CC is likewise analyzed, but cells are then kept in serum-free medium and treated twice during a 48-hour period. |
| Animal Admin | A subcutaneous A549 xenograft model in nude mice is used to evaluate the anticancer activity of CP-673451. Briefly, A549 cells are injected into the axillary regions of mice (2×106 cells/mouse). When the tumor volumes reach 70 mm3, the mice are randomly assigned to a control group and two CP-673451 groups (n=6 per group): low-dose (20 mg/kg) and high dose (40 mg/kg) groups (vehicle 10% 1-methyl-2-pyrrolidinone and 90% polyethylene glycol 300). These animals are administered intraperitoneally with CP-673451 (20 or 40 mg/kg/day) or with vehicle. During the treatment period, the implanted tumors are measured by caliper once a day in a blind fashion. The animal body weights are also measured at the same time. The tumor volume is calculated. After treatment, the mice are killed, and the tumors are harvested and analyzed. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 656.3±65.0 °C at 760 mmHg |
| Molecular Formula | C24H27N5O2 |
| Molecular Weight | 417.504 |
| Flash Point | 350.7±34.3 °C |
| Exact Mass | 417.216461 |
| PSA | 78.43000 |
| LogP | 2.77 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.677 |
| Storage condition | -20℃ |